
Dismay as FDA blocks Regenxbio's Hunter syndrome therapy
The FDA said it needs more information before it can approve Regenxbio's gene therapy for Hunter syndrome, in a devastating decision for patients.
Newsletters and Deep Dive digital magazine

The FDA said it needs more information before it can approve Regenxbio's gene therapy for Hunter syndrome, in a devastating decision for patients.

Is patient-centred trial design becoming a strategic advantage for mid-size pharma, helping cut risk, improve timelines, and engage patients?

The Trump administration's long-promised direct-to-consumer (DTC) sales channel for medicines, TrumpRx, has finally gone live.
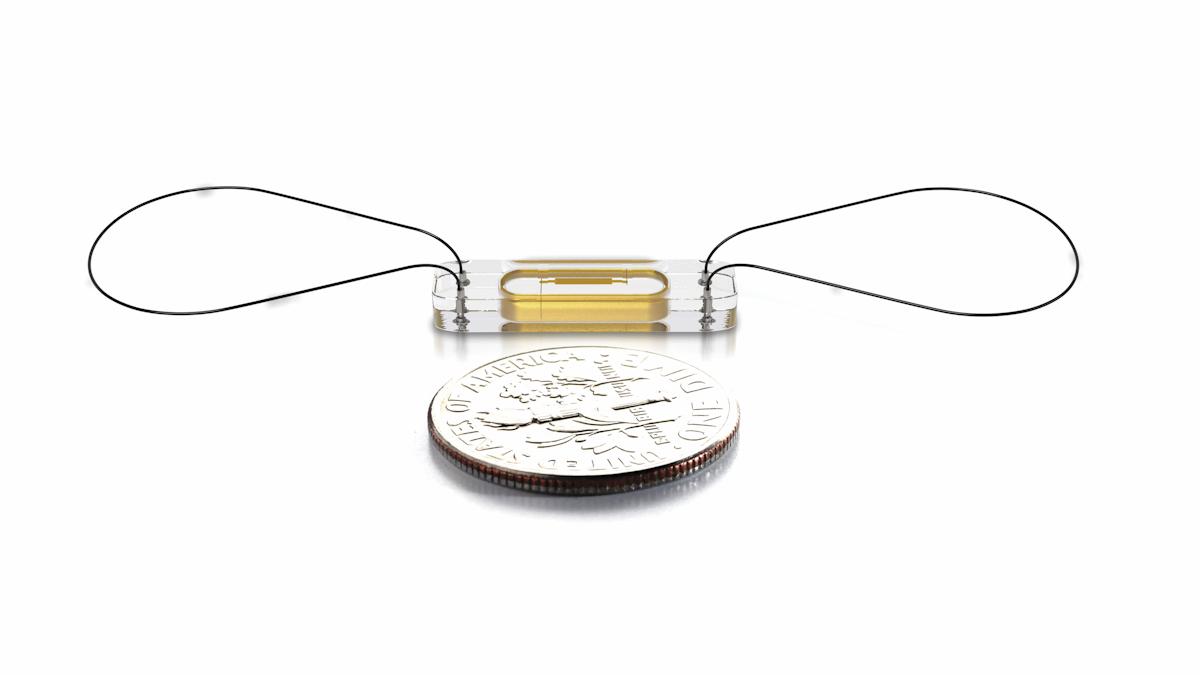
A wireless sensor the size of a paperclip, Abbott's CardioMEMS HF, can now be used routinely by the NHS to monitor patients with heart failure.

The UK confirms that the cost of the drug pricing deal with the US, initially around £1bn over three years, will be borne by the health department.
Editor's Picks
Newsletters and Deep Dive
digital magazine