
Hims & Hers copies Novo Nordisk's new Wegovy pill
Competition has already arrived for Novo Nordisk's Wegovy pill, as telehealth company Hims & Hers launches a compounded version.
Newsletters and Deep Dive digital magazine

Competition has already arrived for Novo Nordisk's Wegovy pill, as telehealth company Hims & Hers launches a compounded version.

Amgen confirmed on its fourth-quarter results call that it has resisted a request to pull Tavneos for ANCA-associated vasculitis from the US market.
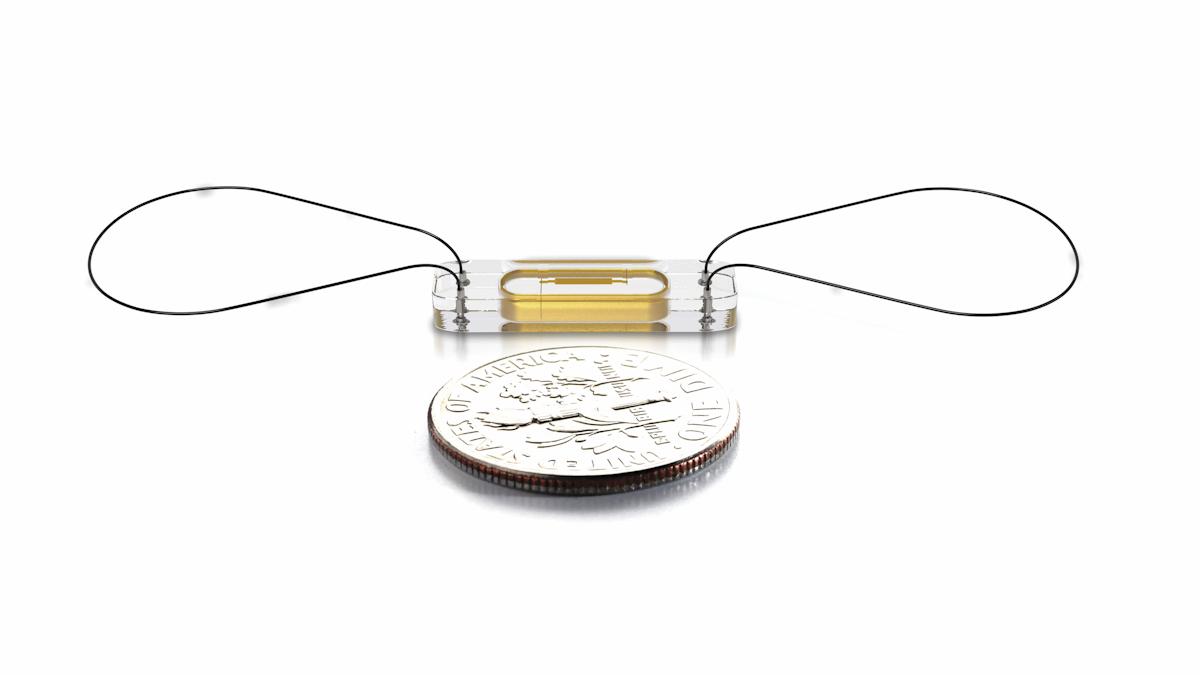
A wireless sensor the size of a paperclip, Abbott's CardioMEMS HF, can now be used routinely by the NHS to monitor patients with heart failure.

At the Biotech Showcase during JPM Week, biotech leaders expressed frustration at the US's lack of concern about Chinese biotech progress.

The UK confirms that the cost of the drug pricing deal with the US, initially around £1bn over three years, will be borne by the health department.
Editor's Picks
Newsletters and Deep Dive
digital magazine