
Flagship's AI-focused Generate Biomedicines files IPO
Generate Biomedicines has filed for an IPO on the Nasdaq, seeking to raise upwards of $100m for phase 3 trials of its twice-yearly asthma drug.
Newsletters and Deep Dive digital magazine

Generate Biomedicines has filed for an IPO on the Nasdaq, seeking to raise upwards of $100m for phase 3 trials of its twice-yearly asthma drug.
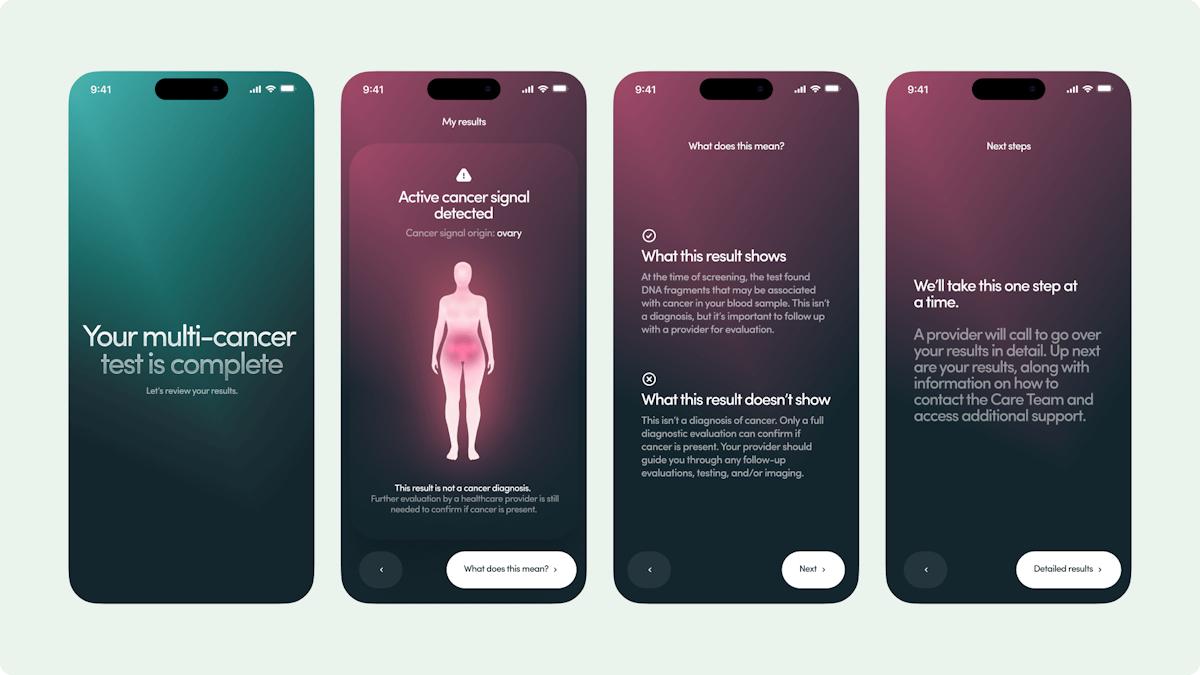
Grail has teamed up with telehealth company Hims & Hers to boost access to its recently filed Galleri blood test for cancer screening.

Eikon and Veradermics push upsized IPOs over the line, as Salspera, which is developing live microbial immunotherapies for cancer, seeks a listing.

Immuneering's Ben Zeskind explains how, by focusing on quality of life as well as quantity, they've created a drug for pancreatic cancer.

FDA has delivered a setback to AstraZeneca by rebuffing its new formulation of lupus therapy Saphnelo, which was approved in Europe last year.
Editor's Picks
Newsletters and Deep Dive
digital magazine