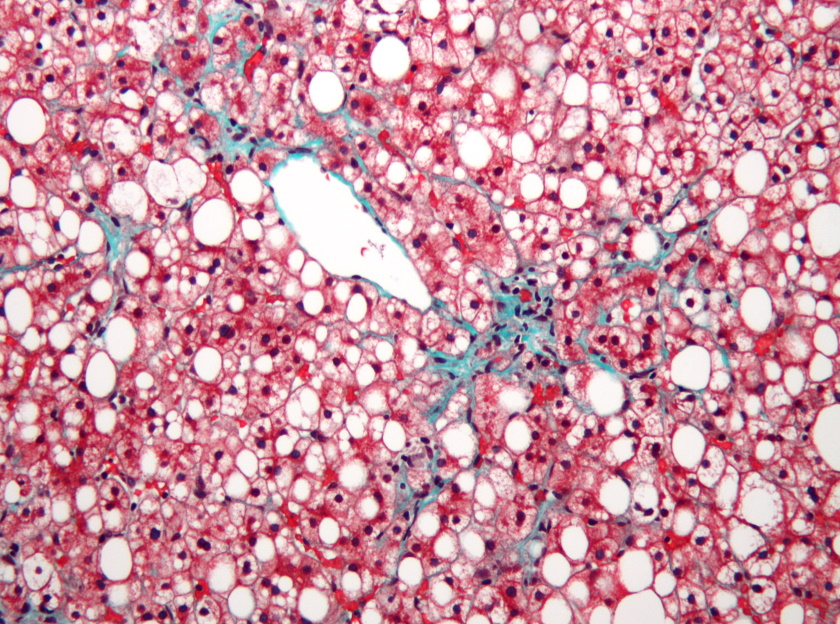
NGM craters after dropping mid-stage NASH candidate aldaferm...
Shares in NGM Biopharma have plummeted after the US biotech said it would abandon development of aldafermin in non-alcoholic steatohepatitis (NASH), adding to a lengthening list of failed c







