Xenotransplantation at the brink of breakthrough: A new era in organ transplantation
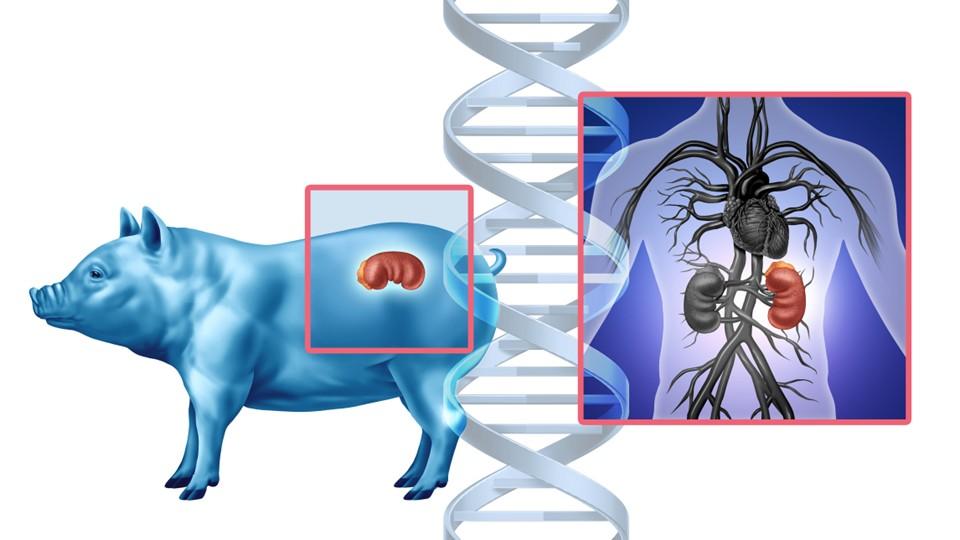
In a landmark procedure performed at Massachusetts General Hospital (MGH) on 25th January 2025, a 66-year-old man with end-stage renal disease (ESRD) received a genetically modified pig kidney, marking the second successful xenotransplant of its kind. The patient, discharged just one week post-surgery, was subsequently able to come off dialysis for the first time in over two years – an outcome that underscores the transformative potential of xenotransplantation in addressing the global organ shortage crisis.
This milestone was made possible through a novel immunosuppressive regimen featuring tegoprubart, an investigational anti-CD40L antibody developed by Eledon Pharmaceuticals, which is designed to inhibit the CD40L signalling pathway, a central mechanism in transplant rejection, offering a targeted and potentially safer alternative to traditional immunosuppressants.
Since then, Eledon has presented new positive data from an ongoing Phase 1b trial evaluating tegoprubart for prevention of rejection in kidney transplantation, the results of which were presented at the World Transplant Congress (WTC) in San Francisco in August. Kidney function, as measured by estimated glomerular filtration rate (eGFR), stabilised after the first month post-transplant and preliminary iBox data - a key biomarker of kidney function and immunologic response - supports that tegoprubart may improve five-year graft survival vs. current SoC. There have been no cases of death, graft loss, drug-related tremor or new-onset diabetes, which are side effects often associated with SoC immunosuppression therapy.
To find out more, pharmaphorum spoke with Dr David-Alexandre Gros, Eledon’s CEO.
A history of innovation and urgency
Xenotransplantation, the transplantation of organs or tissues between species, has a long and complex history. From early experiments in the 17th century to the first transgenic pigs in the 1990s, the field has evolved in response to both scientific advancement and the pressing need for donor organs. The use of pigs, particularly genetically modified ones, has emerged as the most viable option, due to their physiological compatibility, rapid growth, and reduced ethical concerns compared to primates.
Recent developments in this field have focused on overcoming immunological barriers. As Dr Gros explained: “Preclinical studies in the last five years have shown that the likely most effective way to prevent xenotransplant rejection is to block the CD40 ligand (CD40L), which is what our investigational drug tegoprubart is designed to do.”
Tegoprubart’s mechanism of action targets the CD40L pathway, which plays a pivotal role in immune cell communication. By inhibiting this pathway, tegoprubart reduces the activation of helper T-cells, killer T-cells, and B-cells – all key players in organ rejection – while paradoxically enhancing regulatory T-cells (Tregs), which suppress immune responses.
This targeted approach offers several advantages over conventional immunosuppressants, which often cause significant side effects such as nephrotoxicity, hypertension, and diabetes.
“Our approach is more targeted than standard of care immunosuppressants that aim to wipe out populations of white blood cells,” Dr Gros noted, “and has demonstrated the potential for better efficacy and improved safety.”
A turning point in transplant medicine
The latest xenotransplant at MGH is not an isolated success. Tegoprubart has now been used in three major xenotransplant procedures: two kidney transplants at MGH and a heart transplant at the University of Maryland Medical Center. These cases represent incremental but meaningful steps toward a future where organ availability and longevity are no longer limiting factors.
“This second kidney xenotransplant conducted at MGH represents another important milestone,” said Dr Gros. “We are now at the technological forefront of potentially achieving two groundbreaking objectives: significantly extending organ viability post-transplant (‘one organ for life’) and universal availability of organs (‘one organ for all’).”
The urgency of innovation in transplantation is underscored by sobering statistics: for every five patients who receive a kidney transplant, one dies waiting. Moreover, most transplanted kidneys fail within 10–15 years, necessitating repeat transplants and further straining the organ supply.
Eledon’s dual approach – developing better immunosuppressive therapies and enabling new organ sources through xenotransplantation – aims to address these challenges head-on.
“By collaborating with MGH and other top-tier institutions, we hope to show that transplantation using our novel immunosuppression drug is one of the key viable solutions to solving the organ shortage,” Dr Gros stated.
Looking ahead: A vision for transplantation
Eledon’s vision is bold, yet grounded in clinical progress.
“Our hope is that tegoprubart will be the next cornerstone immunosuppression therapy in transplantation, regardless of the transplant type – whether kidney, heart, lung, or islet cell – and regardless of the source of the organ,” Dr Gros affirmed.
With ongoing clinical trials and promising early data, tegoprubart may soon redefine the standard of care in transplantation medicine. Its potential to improve both patient survival and quality of life positions it as a beacon of hope in a field long constrained by biological and logistical limitations.
Xenotransplantation, once a distant aspiration, is now a tangible frontier in modern medicine. Through pioneering work at institutions like MGH and innovative therapies like tegoprubart, the dream of universal, long-lasting organ transplants is closer than ever. As research continues and collaboration deepens, the healthcare community stands on the cusp of a new era – one where no patient dies waiting for an organ, and every transplant offers a lifetime of function.
About the interviewee
 Dr Gros has served as CEO and a member of the Board of Directors of Eledon Pharmaceuticals, Inc. (formerly Novus Therapeutics, Inc.) since September 2020. He joined Eledon Pharmaceuticals from Imbria Pharmaceuticals Inc., where he served as co-founder, CEO, and Chairman of the Board of Directors. Prior to Imbria, Dr Gros was president and chief operating officer of Neurocrine Biosciences, Inc., chief business and principal financial officer of Alnylam Pharmaceuticals, Inc., and chief strategy officer of Sanofi, SA. Before joining Sanofi, Dr Gros held leadership positions in healthcare investment banking at Centerview Partners LLC, and Merrill Lynch, Pierce, Fenner & Smith Inc., and in healthcare consulting at McKinsey & Company. He previously served on the Board of Directors of Eliem Therapeutics, Inc., a biotechnology company he co-founded, and of Saint Jean Groupe, SA, a leading French manufacturer of pasta products since 1935. Dr Gros earned his Doctor of Medicine from The Johns Hopkins University School of Medicine, a Master of Business Administration from Harvard Business School, and a Bachelor of Arts from Dartmouth College.
Dr Gros has served as CEO and a member of the Board of Directors of Eledon Pharmaceuticals, Inc. (formerly Novus Therapeutics, Inc.) since September 2020. He joined Eledon Pharmaceuticals from Imbria Pharmaceuticals Inc., where he served as co-founder, CEO, and Chairman of the Board of Directors. Prior to Imbria, Dr Gros was president and chief operating officer of Neurocrine Biosciences, Inc., chief business and principal financial officer of Alnylam Pharmaceuticals, Inc., and chief strategy officer of Sanofi, SA. Before joining Sanofi, Dr Gros held leadership positions in healthcare investment banking at Centerview Partners LLC, and Merrill Lynch, Pierce, Fenner & Smith Inc., and in healthcare consulting at McKinsey & Company. He previously served on the Board of Directors of Eliem Therapeutics, Inc., a biotechnology company he co-founded, and of Saint Jean Groupe, SA, a leading French manufacturer of pasta products since 1935. Dr Gros earned his Doctor of Medicine from The Johns Hopkins University School of Medicine, a Master of Business Administration from Harvard Business School, and a Bachelor of Arts from Dartmouth College.