Formedix Partnered with CDISC to Give Free Access to CDASH-Compliant eCRFs

Formedix and CDISC Join Forces to Provide Free Access to Ready-to-Use, CDASH-Compliant eCRFs for Accelerated Clinical Trial Set-Up Formedix, a leading provider of clinical trial software solutions, has collaborated with CDISC, the global clinical research data standards development organization, to create a novel portal providing free access to ready-to-use, CDASH-compliant, annotated electronic case report forms (eCRFs). As part of this partnership, Formedix’s innovative all-in-one cloud-based clinical metadata repository (CMDR) and study automation platform — ryze — is being used to design CDASH-compliant eCRFs. Forms can be visualized in real time in ryze, before being easily exported into any of the leading electronic data capture (EDC) systems, expediting the clinical trial set-up process. 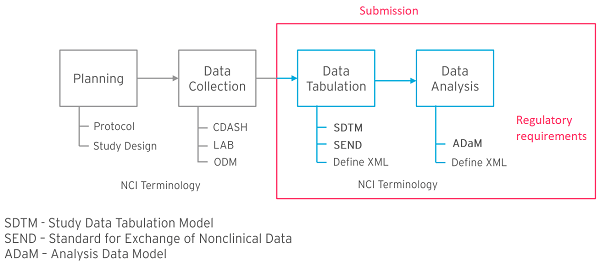 “Formedix has more than two decades of experience in CDISC standards development. We contributed to the creation of the CDISC ODM, Define and Dataset-XML models, and we are also a member of the CDISC Data Exchange Standards team,” said Kevin Burges, Head of Product Management at Formedix. “We are very proud to be able to extend this long-standing partnership, and use our expertise to provide pharmaceutical, biotechnology and CRO organizations with an out-of-the-box solution that accelerates eCRF development, while also promoting data quality and reuse for better interoperability.” Through the new collaboration, CDISC users can access the unique capability of ryze to design and visualize eCRFs, as well as making any necessary changes to forms, without having to build their EDC first. As a result, clinical trial set-up can take as little as 6 weeks. Furthermore, the eCRFs are CDASH-compliant, which facilitates submission to regulatory authorities. Importantly, ryze is a commercial off-the-shelf system, meaning it is available for use immediately and can be rapidly configured to meet specific application needs. “We are using the Formedix platform to design CDASH-compliant, example CRFs for the CDISC eCRF Portal,” noted Peter Van Reusel, CDISC Chief Standards Officer. “We can create, visualize, edit and approve eCRFs in the platform, then export them in ODM-XML to use in the CDISC Library and standards packages. Formedix is one of CDISC’s longest standing members. CDISC is keen to work with technology partners that help pharmaceutical organizations to adopt and keep pace with emerging CDISC standards.” Designed for ease-of-use and to align with varying application requirements, the eCRFs within the CDISC portal are available in multiple formats, including PDF, HTML and XML. To access the CDISC eCRF portal, please visit www.cdisc.org/kb/ecrf.
“Formedix has more than two decades of experience in CDISC standards development. We contributed to the creation of the CDISC ODM, Define and Dataset-XML models, and we are also a member of the CDISC Data Exchange Standards team,” said Kevin Burges, Head of Product Management at Formedix. “We are very proud to be able to extend this long-standing partnership, and use our expertise to provide pharmaceutical, biotechnology and CRO organizations with an out-of-the-box solution that accelerates eCRF development, while also promoting data quality and reuse for better interoperability.” Through the new collaboration, CDISC users can access the unique capability of ryze to design and visualize eCRFs, as well as making any necessary changes to forms, without having to build their EDC first. As a result, clinical trial set-up can take as little as 6 weeks. Furthermore, the eCRFs are CDASH-compliant, which facilitates submission to regulatory authorities. Importantly, ryze is a commercial off-the-shelf system, meaning it is available for use immediately and can be rapidly configured to meet specific application needs. “We are using the Formedix platform to design CDASH-compliant, example CRFs for the CDISC eCRF Portal,” noted Peter Van Reusel, CDISC Chief Standards Officer. “We can create, visualize, edit and approve eCRFs in the platform, then export them in ODM-XML to use in the CDISC Library and standards packages. Formedix is one of CDISC’s longest standing members. CDISC is keen to work with technology partners that help pharmaceutical organizations to adopt and keep pace with emerging CDISC standards.” Designed for ease-of-use and to align with varying application requirements, the eCRFs within the CDISC portal are available in multiple formats, including PDF, HTML and XML. To access the CDISC eCRF portal, please visit www.cdisc.org/kb/ecrf.











