Are European incentives speeding access in rare cancer treatments?
To help orphan drugs reach patients, the EMA provides drug developers with R&D, regulatory and financial help. Himakshi Sharma examines the impact of this approach.
In Europe, heterogeneity in health technology assessment (HTA) across European Union (EU) member states means there is variability in the availability of, and access to, drugs. Improving access to orphan drugs – particularly those for rare cancers – is a crucial decision and one which HTA attempts to simplify.1
Orphan medicinal products
The EU regulation on Orphan Medicinal Products (OMP) confers orphan designation to products for conditions that affect not more than five in 10,000 people in the EU and which are unlikely to be marketed without incentives.2 The European Medicines Agency (EMA) received 2,302 such applications between the introduction of the regulation in 2000 and September 2015, of which its Committee for Orphan Medicinal Products (COMP) approved 1,544. About a third of these were for some form of rare cancer.3
The EMA provides incentives to sponsors seeking marketing authorisation (MA) for products designated as orphan drugs, as depicted in Figure 1.4
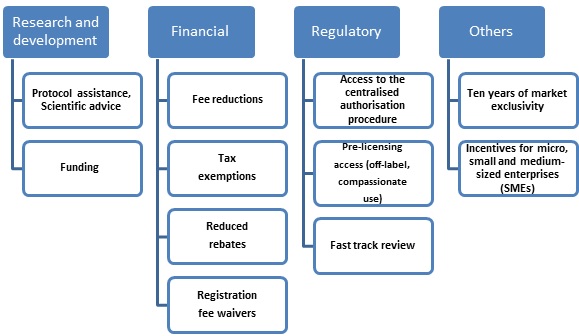
Figure 1: Types of incentive provided by the EMA4
Recent success stories
There are many examples where incentives have helped improve access to drugs for rare cancers in Europe.
A recent one is the addition of ‘follicular lymphoma’ to Gazyvaro’s (obinutuzumab) approved indications. It was first granted MA in 2014 for previously untreated chronic lymphocytic leukaemia (in combination with chlorambucil). Now the product will be available in combination with bendamustine for patients with follicular lymphoma who were previously treated with chemotherapy. Gazyvaro was designated an orphan medicine in 2015. The sponsors received scientific advice from the Committee for Medicinal Products for Human Use (CHMP) on the design of the phase 3 trial supporting the efficacy and safety of the product, which facilitated MA.5
Many drugs have been approved recently for their ‘orphan designation’ to address unmet cancer treatment needs, including Debiopharm International SA’s IAP inhibitor Debio 1143 (for ovarian cancer), SELLAS Life Sciences Group’s WT1 cancer vaccine (galinpepimut-S) (for acute myeloid leukaemia and malignant pleural mesothelioma). This makes them eligible for financial incentives such as fee waivers and tax rebates, as well as other incentives such as 10-year period of marketing exclusivity in the EU after product approval.6,7
Other improvements in access for cancer drugs are the centralised authorisation procedures, fast-track reviews and pre-licensing access programmes, such as ‘compassionate use’. Nivolumab was approved on compassionate grounds in Ireland recently, benefiting 200 patients with advanced lung cancer for 30 days, free of charge to the patients.8
Similar programmes are being set up in other countries in the EU (e.g. Austria, Estonia, Greece, Hungary, Italy, Lithuania, Portugal, and Spain).9 They have adopted various policies for compassionate use, including named patient programmes for individual patients and complete reimbursement of the medicinal product under compassionate use (such as in Greece).3
Are incentives enough?
Incentives have radically improved MA for orphan drugs for rare cancers, but are they enough?
One major concern after receiving MA is pricing and reimbursement as cost-effectiveness and the decisions on coverage and reimbursements are addressed at national level.10
A recent controversy concerning this was the rejection of Imbruvica (ibrutinib) by the National Institute for Health and Care Excellence (NICE) in England. The drug had acquired the CHMP’s favourable opinion on orphan designation in 2012 for the treatment of chronic lymphocytic leukaemia. This was contradictory to the decision by other EU countries, such as Greece, which accepted the drug under the national reimbursement scheme.11,12
Jakavi (ruxolitinib) received orphan drug designation in 2008 and 2009. However, it was not covered under reimbursement policies in East European countries such as Poland and the Czech Republic until 2015.13,14
Conclusion
So, incentives can help increase access to orphan drugs for rare cancers across the EU. However, improvements to MA are hindered by the unpredictability of pricing and reimbursement decisions at country level. The various stakeholders need to take a more proactive approach to close the gap between regulatory approval and reimbursement decisions. They can benefit from adopting adaptive licensing, risk-sharing agreements, conditional coverage and patient registries that can provide data on local resource utilisation, safety, effectiveness, and costs.
Thus the authorities are better equipped to forecast and manage already stressed budgets. Physicians can use the data to continuously monitor disease progression, compare treatment in the real-world setting and make informed decisions on sub-populations and concomitant medications. Patients are assured access to novel and otherwise unaffordable medications through a defined process, and pharma companies have a continuous flow of data that could support submissions for complete reimbursement in the future.
References:
- Tordrup D, Tzouma V, Kanavos P. Orphan drug considerations in Health Technology Assessment in eight European countries. Rare Diseases and Orphan Drugs Journal. 2014;1 (3):86-97
- Committee for Orphan Medicinal Products – strengthened interactions with patients and international partners. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/02/news_detail_001718.jsp&mid=WC0b01ac058004d5c1. Accessed on 13 June 2016.
- Inventory of Union and Member State incentives to support research into, and the development and availability of, orphan medicinal products. STATE OF PLAY 2015. Available from http://ec.europa.eu/health/files/orphanmp/doc/orphan_inv_report_20160126.pdf. Accessed on 13 June 2016.
- Orphan incentives. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000393.jsp. Accessed on 13 June 2016.
- New treatment for rare white blood cell cancer. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/04/news_detail_002522.jsp&mid=WC0b01ac058004d5c1. Accessed on 13 June 2016.
- EMA grants Orphan Drug Designation to Debiopharm International SA’s IAP inhibitor Debio 1143 in the treatment of Ovarian Cancer. Available from https://www.debiopharm.com/component/k2/item/3600-ema-grants-orphan-drug-designation-to-debiopharm-international-sa-s-iap-inhibitor-debio-1143-in-the-treatment-of-ovarian-cancer.html. Accessed on 13 June 2016.
- SELLAS Life Sciences Group Receives a Favorable Opinion for European Orphan Drug Designations for WT1 Cancer Vaccine (galinpepimut-S) for the Treatment of Acute Myeloid Leukemia and for Malignant Pleural Mesothelioma. Available from http://www.prnewswire.com/news-releases/sellas-life-sciences-group-receives-a-favorable-opinion-for-european-orphan-drug-designations-for-wt1-cancer-vaccine-galinpepimut-s-for-the-treatment-of-acute-myeloid-leukemia-and-for-malignant-pleural-mesothelioma-300246269.html. Accessed on 13 June 2016.
- New cancer drug to be given to patients free for 30 days. Available from http://www.irishtimes.com/news/health/new-cancer-drug-to-be-given-to-patients-free-for-30-days-1.2668501. Accessed on 13 June 2016.
- Expanded Access Program (Compassionate Use) Investigator Requests. Available from http://www.bms.com/clinical_trials/investigator_sponsored_research/Pages/expanded-access-program.aspx. Accessed on 13 June 2016.
- An overview of the incentives in the EU and USA and their drawbacks to promote drugs for the treatment of rare diseases. Available from http://dgra.de/deutsch/studiengang/master-thesis/2012-Laurent-Schmitt-An-overview-of-the-incentives-in-the-EU-and-USA-and-their-draw-b?nav=studiengang
- NICE's rejection of Imbruvica 'reveals appraisal process flaws'. Available from http://www.pmlive.com/pharma_news/nices_rejection_of_imbruvica_reveals_flaws_in_appraisal_process_947489. Accessed on 13 June 2016.
- CHMP assessment report. Imbruvica. 24 July 2014. Available from http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003791/WC500177777.pdf. Accessed on 13 June 2016.
- Summary of opinion (initial authorisation). Jakavi. 19 April 2012. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002464/WC500125696.pdf. Accessed on 13 June 2016.
- Access to innovative cancer drugs in Poland in comparison with selected European Union countries and Switzerland, 2015. Report ordered by Young People Oncology Foundation Alivia and prepared by EY Poland. Available from https://www.alivia.org.pl/raport2015/05_05_2015_Raport_wersja_EN.pdf. Accessed on 13 June 2016.
About the author:
Dr Himakshi Sharma is a Senior Scientific Associate at Phamax AG.
A dentist and a postgraduate diploma holder in Clinical Research and Pharmacovigilance, she is experienced in medical writing and medical communications.
Prior to joining Phamax, she was a medical writer for pharma companies in Europe and the USA and has also worked in medical communications.
Read more from Phamax:
We can eradicate Hepatitis C in the emerging world - here's how