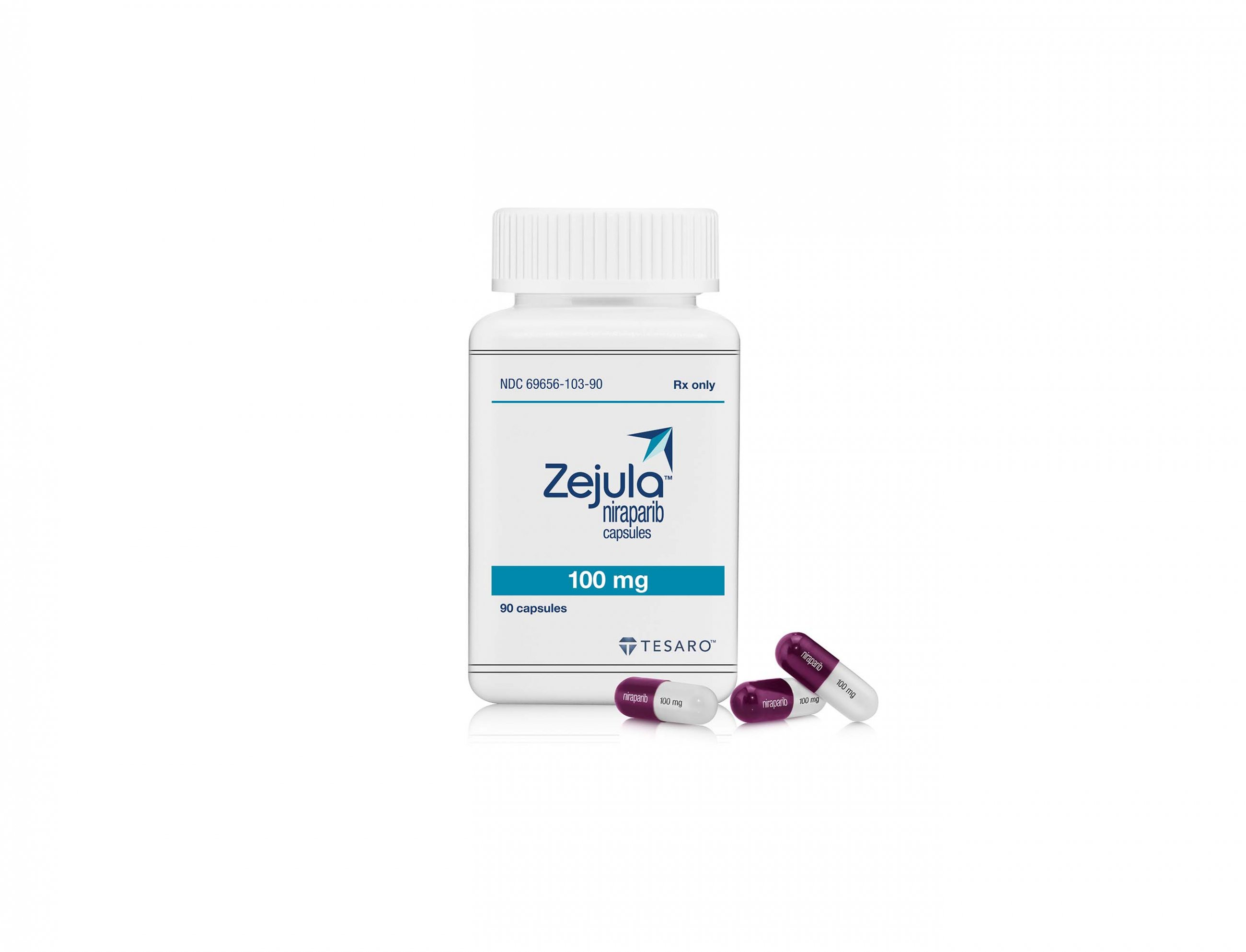
GSK hits another oncology hurdle as Zejula use is limited ag...
GSK has had another setback in its oncology business, after the FDA asked it to restrict use of its PARP inhibitor Zejula in ovarian, fallopian tube, or primary peritoneal cancer to patient