Solution Spotlight: Accelerator Drug Development

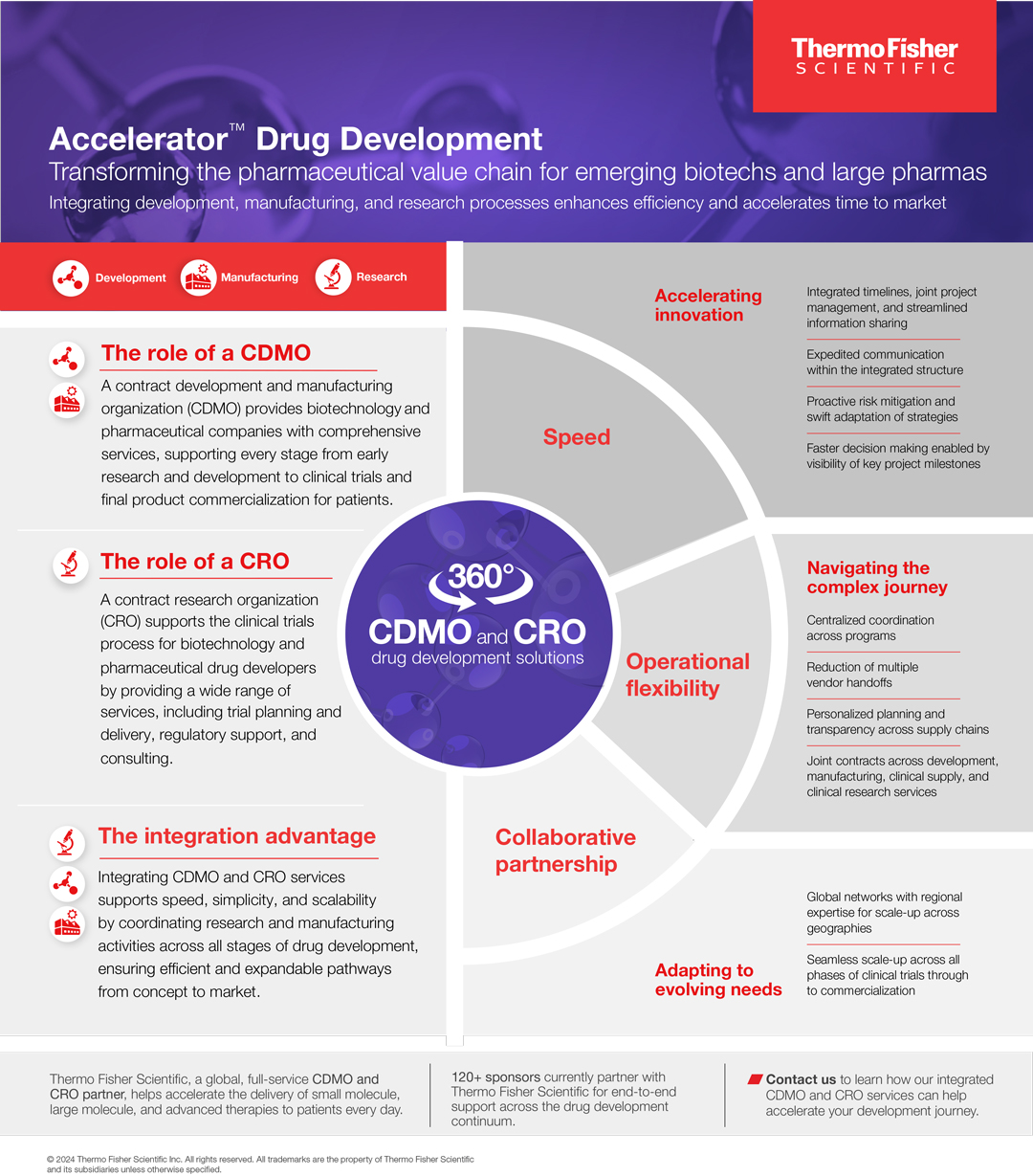
Thermo Fisher Scientific’s Accelerator Drug Development is a suite of integrated services combining CDMO and CRO capabilities to streamline the drug development journey, from early-stage research to commercialisation. As a single, connected partner for clinical research, manufacturing, and clinical supply chain management, we can drive greater efficiency and mitigate risks, enabling companies to get their new medicines and therapies to market faster, across all major therapeutic areas and drug modalities.
What problem or status quo deficiency are you solving?
Due to escalating development costs, increasing scientific and logistical demands, regulatory complexity, and mounting pressure to accelerate timelines, drug developers are facing unprecedented challenges in bringing their new therapies to market. The traditional reliance on multiple vendors to manage different aspects of development – from drug substance and drug product manufacturing to clinical research and clinical supply – further compounds these challenges. Relying upon this fragmented model often introduces inefficiencies, miscommunication, and delays, making it even more difficult for companies to navigate an already complex landscape.
What innovations does your solution bring to bear on these challenges?
By unifying manufacturing, clinical research, clinical laboratories, and clinical supply chain services through a single partner, pharma and biotech organisations of all sizes now have the ability to realise a new value equation – one that reduces handovers and white space, prioritises speed, efficiency, and strategic advantage in an increasingly complex landscape. This paradigm shift in how drugs are developed is designed to reduce risk, streamline operations, and accelerate the path to market, ultimately delivering greater impact for patients.
How does your solution work?
As we understand every drug development journey is unique, customers can partner with Thermo Fisher at any point, in any phase of their drug development, and gain value by utilising combinations of our CDMO and CRO services. To deliver on this promise, each project is supported by a dedicated integrated program management team providing centralised oversight and accountability to ensure strategic objectives and key milestones are met. Specific mechanisms for driving acceleration include integrated project timelines and information sharing, innovative digital and AI-powered tools to drive efficiency within key service areas, proactive risk mitigation strategies, personalised planning and transparency across supply chains, and faster decision making enabled by shared visibility to key project milestones.
What makes your solution stand out in the market?
While many service providers may offer “integrated” or “end-to-end” solutions, Thermo Fisher provides a leading offering with a full suite of CDMO, CRO, and clinical supply services to support preclinical to commercial drug development. Our customers benefit from the collective experience we have gained from our long tenure in the industry, the scale and diversity of programmes supported, and the depth and breadth of services offered. We draw upon our extensive resources and deep expertise to understand each customer’s unique challenges, designing a customised approach that meets their specific needs regardless of project size or phase of development.
What concrete outcomes and KPI benefits does your solution deliver?
Research findings from the Tufts Center for the Study of Drug Development validate that integrating services under a single provider significantly accelerates timelines to generate measurable financial impact. The Tufts research team used a model called expected net present value (eNPV) to assess the impact of this integrated approach on oncology programmes. eNPV looks at the costs of development, weighs in the risks, and compares them against potential future revenues, with a positive score indicating a programme is worthy of investment. When the Tufts team applied the model using both industry data and operational metrics from clinical trials managed by Thermo Fisher, they observed up to $63m in added financial return and 113x ROI for mAbs, and up to $25m in added financial return and 47x ROI for small molecules. Additionally, a seven–34 month reduction in development timelines was observed when using both CDMO and CRO services.
How does your solution improve the experience for the end user?
Accelerator Drug Development is designed to overcome common pitfalls of the traditional, multi-vendor outsourcing model in drug development, such as inefficiencies, miscommunication, and timelines delays. These systemic challenges, often rooted in coordination gaps, data silos, and fragmented processes, compound the escalating costs of drug development and the complexities of navigating a stringent global regulatory landscape. With our solution, customers can focus less on coordination and more on high value strategic activities to drive innovation. Ultimately, Accelerator Drug Development is both improving the day-to-day customer experience while supporting their aspirations to get their treatments to patients faster.
About Thermo Fisher Scientific

Thermo Fisher Scientific is the world leader in serving science, with annual revenue over $40 billion. Our mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating life sciences research, solving complex analytical challenges, increasing productivity in their laboratories, improving patient health through diagnostics or the development and manufacture of life-changing therapies, we are here to support them. Our global team delivers an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, Patheon, and PPD. For more information, please visit thermofisher.com.