The AI imperative: Establishing the standard for modern clinical research

The clinical trial industry is at a critical juncture where traditional methods are proving insufficient for managing today’s complex studies.
AI is rapidly emerging as an essential strategic tool to maintain competitiveness and control rising costs. Analysis shows that the incorporation of AI is gaining significant momentum across the sector. Already, 56% of organisations are either actively using AI in some trials or are in the process of adopting it. This accelerated integration is driven by tangible results: 73% of AI users report that the technology has met or exceeded their expectations, fuelling continued progress. The pathway to future development success requires integrated, intelligent ecosystems.
Evaluating early success: Where AI is delivering tangible value
The findings from the Medidata-commissioned survey confirm that AI implementation in clinical trials has moved firmly beyond theoretical discussion into core operational functions.
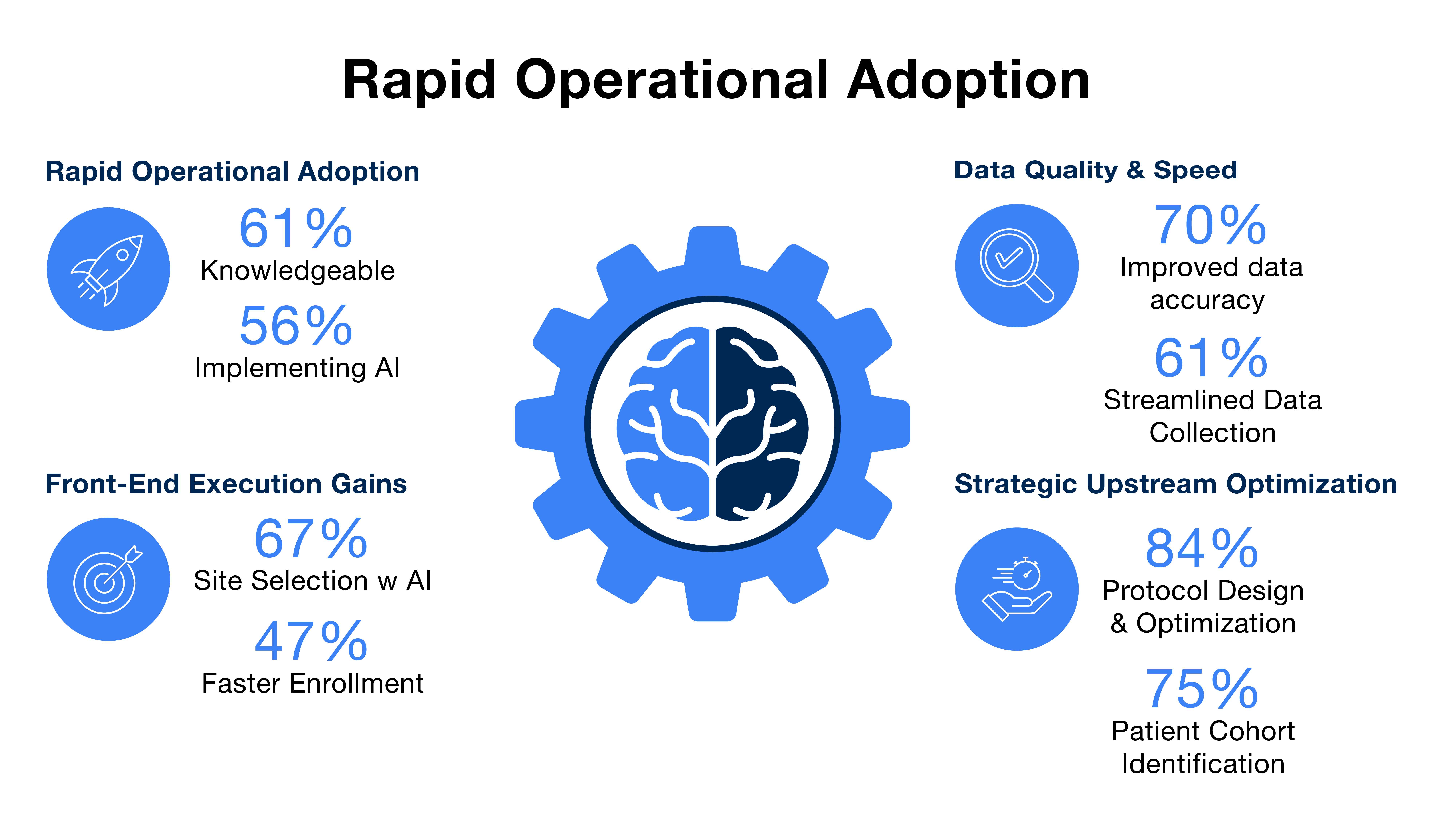
- Rapid operational adoption: Widespread knowledge (60% of all participants are 'moderately' or 'extremely' knowledgeable) supports rapid implementation, with 56% of companies already using AI or actively implementing it.
- Focus on data quality and speed: AI is predominantly used in data capture and quality oversight (over 70% of users) to manage data complexity. Benefits include 70% reporting improved data accuracy and 61% citing streamlined data collection.
- Strategic upstream impact: Adoption is strong in de-risking and accelerating development: 84% of AI users employ the technology for protocol design and optimisation, and 75% for patient cohort identification.
- Front-end execution gains: Two-thirds of users (67% overall) currently use AI for site feasibility and selection, with nearly half (47%) reporting improved site selection for faster enrolment.
Beyond design and data handling, AI is playing a critical role in essential regulatory and operational functions. For instance, CSR (Clinical Study Report) preparation and submission is a high-use area, with 72% of AI user respondents indicating their companies employ AI here. In quality management, 62% of respondents indicated that their companies are using AI for Risk-Based Quality Management (RBQM). Furthermore, AI-powered tools are augmenting site capabilities, with over half of AI users (53%) utilising the technology for personalised patient communications/chatbots and 54% for automated visit scheduling and reminders.
The overall satisfaction with AI is a compelling finding: 73% of AI users reported that the technology has met or surpassed their expectations. This satisfaction indicates that AI is successfully identifying potential issues earlier, allowing teams to focus on critical thinking and decision-making, rather than being overwhelmed by data demands.
The next horizon: Expected growth areas for AI in the coming year
There is, then, a clear forecast for the immediate strategic priorities of clinical organisations in the next 12 months, with areas ripe for growth for both current AI users and those planning initial adoption. Focus will be on high-value, resource-intensive activities, particularly those that ensure data quality and accelerate reporting.
Among current AI users, the highest area for expected utilisation is CSR (Clinical Study Report) preparation and submission, with 59% planning to employ AI in this activity over the next year. This suggests a move toward automating the final stages of regulatory output. Close behind is the continued investment in data quality and integrity functions: 57% of current users expect to use AI for outlier and anomaly detection, and 56% anticipate utilising AI for data capture and data quality oversight.
Crucially, protocol authoring is projected to be a major growth area, with 57% of current AI users planning to implement AI here, despite its lower current adoption rate compared to protocol design. This indicates a growing realisation that optimising protocol language using AI will minimise costly amendments and delays.
For the segment of the industry currently categorised as non-AI users (those not yet actively using AI in trials), the focus for 2026 is on establishing foundational capabilities. Nearly half of these non-users (48%) anticipate adopting AI for data visualisation within the next 12 months, making it the most likely entry point for new adopters.
Several other core infrastructure and planning functions are poised for growth among non-AI users, with approximately one-third expecting implementation in key areas. This includes protocol development & authoring (38% expected use), data management (34% expected use), and data standardisation (34% expected use). The drive toward data standardisation is highly relevant, as AI is proving effective at co-localising and standardising data from multiple disparate sources in real-time, a capability essential for seamless trial execution. Furthermore, 33% of non-users expect to adopt AI for patient engagement, and 30% expect adoption in feasibility and start-up.
These anticipated adoption trends underscore that organisations are moving towards integrating AI across the entire spectrum of clinical trial workflows. With only 7% of participants reporting no plans to incorporate AI within the next 12 months, the industry is approaching a tipping point where failing to accelerate adoption efforts means risking falling further behind in this rapidly evolving environment.
AI in clinical trials: A mandate for strategic success
AI is becoming essential to stay competitive in the face of rising trial complexity. The vast majority of organisations have already started their AI journey, with 56% actively using AI in some trials or currently in the process of adoption.
AI integration is proving highly valuable: 73% of AI users report that the technology has met or exceeded their expectations. Immediate benefits are concentrated in critical areas of data handling and trial planning. Specifically, 70% of users report improved data accuracy, and 61% cite streamlined data collection. Over 70% of users are leveraging AI successfully for protocol design, patient population identification, and data capture/quality oversight.
Looking ahead, significant investment is expected in core functions such as CSR preparation, outlier detection, and protocol authoring. For companies currently not using AI, key areas for initial adoption include data visualisation and data standardisation.
The overall trend indicates that early adopters are gaining a compounding competitive advantage, conducting studies more quickly and at a lower cost. Accelerated AI integration is therefore a mandate for sustained success.
About the author

Dr Jia Chen, PhD, is a Senior Director at Medidata, where she leads the strategy and growth of AI solutions that transform how clinical trials are designed, built, and executed. Her work empowers life sciences organisations to leverage AI for more informed decision-making and accelerated development timelines. Her team's innovations were recently recognised as Best Use of AI in Clinical Trials, highlighting their real-world impact. With a background spanning AI incubation, product leadership, and enterprise innovation at IBM, she spearheaded the launch of global-scale solutions serving millions of users and built transformative platforms at the intersection of data, AI, Healthcare, and Life Sciences. Dr Chen led cross-functional teams across North America and emerging markets, driving innovation in both established and fast-growing ecosystems. She was named one of MIT Technology Review’s Top 35 Innovators (TR35) and recognised as one of IBM’s Top 26 Women Tech Innovators. Dr Chen holds 45 patents and earned her PhD in Physics from Yale University. Her early research was honoured with national awards and named among Science magazine’s “Breakthroughs of the Year”. She served as Chair of the Yale Graduate School Alumni Association and on the Yale Alumni Association Board of Governors.
About Medidata

Medidata is powering smarter treatments and healthier people through digital solutions to support clinical trials. Celebrating 25 years of ground-breaking technological innovation across more than 37,000 trials and 11 million patients, Medidata offers industry-leading expertise, analytics-powered insights, and one of the largest clinical trial data sets in the industry. More than 1 million registered users across approximately 2,300 customers trust Medidata’s seamless, end-to-end platform to improve patient experiences, accelerate clinical breakthroughs, and bring therapies to market faster. A Dassault Systèmes brand (Euronext Paris: FR0014003TT8, DSY.PA), Medidata is headquartered in New York City and has been recognized as a Leader by Everest Group and IDC. Discover more at www.medidata.com.
Listen to our latest podcast, from Dreamers to Disruptors, and follow us at @Medidata.
To find out more read the full report here.











