Novo Nordisk claims first GLP-1 approval in MASH
Novo Nordisk's GLP-1 drugs have been under considerable competitive pressure in the last few months, so the company will be celebrating a first-in-class approval from the FDA for Wegovy in metabolic dysfunction-associated steatohepatitis (MASH).
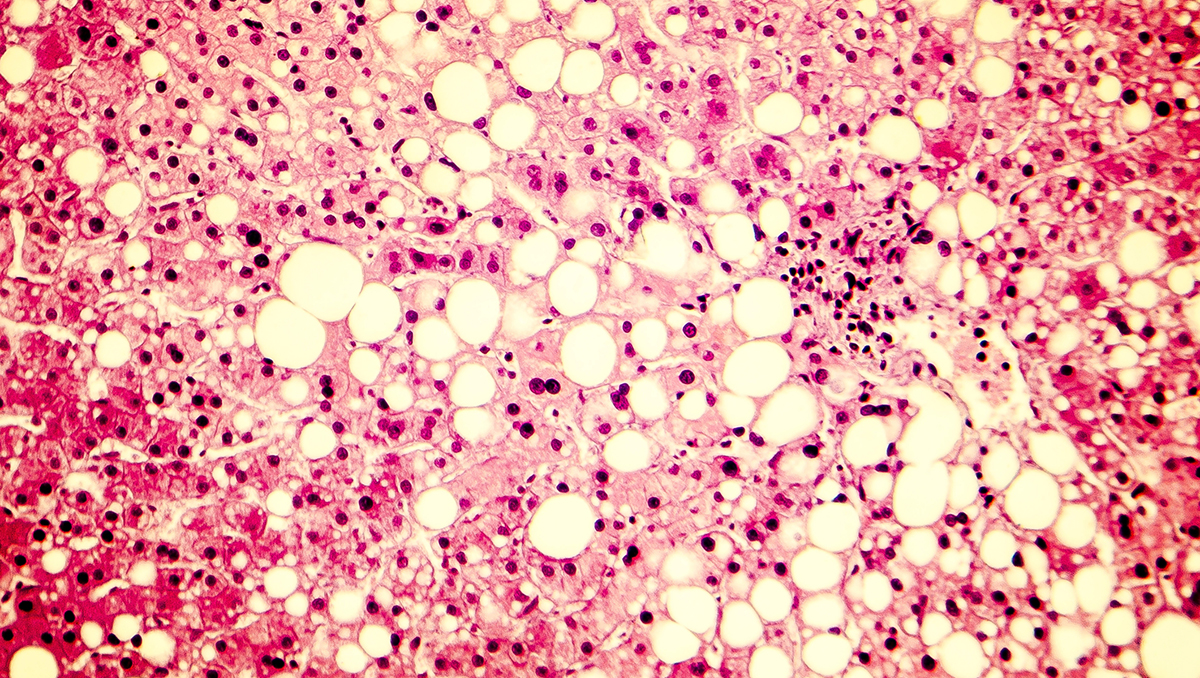
The US regulator has cleared weight-loss drug Wegovy (semaglutide) for patients with MASH and moderate to advanced liver scarring (fibrosis), without cirrhosis, in combination with a reduced-calorie diet and exercise.
The approval could unlock a big new market opportunity for Wegovy, as MASH is a form of non-alcoholic fatty liver disease (NAFLD) that affects millions of people worldwide and has been billed as pharma's next big growth area, with some analysts predicting a market potentially worth tens of billions of dollars a year.
The condition is largely associated with obesity and an unhealthy diet and lifestyle, and is on the rise in industrialised nations.
Wegovy's approval is based on results from Part 1 of the ESSENCE trial, which involved around 800 patients treated with semaglutide or placebo on top of standard care and followed for 72 weeks. All told, 37% of people treated with semaglutide saw an improvement in liver fibrosis with no worsening of steatohepatitis compared to 22.5% on placebo at the 72-week timepoint. Meanwhile, resolution of steatohepatitis with no worsening of liver fibrosis was seen in 62.9% and 34.1% of patients, respectively.
The approval keeps Novo Nordisk's nose in front of its main rival in the GLP-1 market, Eli Lilly, which is also hoping to add MASH to the indications for Wegovy rival Zepbound (tirzepatide), a dual GIP/GLP-1 agonist that has proved better than Wegovy at achieving weight loss in head-to-head trials.
Squeezed in its main obesity market by Zepbound, Wegovy is also being affected by compounding pharmacies working with telehealth companies, which are providing semaglutide to customers under a loophole in the law that allows them to provide 'personalised' doses of the GLP-1 drug.
Previously, the only FDA-approved therapy for MASH was Madrigal Pharma's THR β-selective agonist Rezdiffra (resmetirom), which was cleared in the US last year and looks to be on a path to blockbuster status, with sales reaching around $350 million in the first half of this year. Analysts have suggested that the benefit of semaglutide in trials is similar to that seen with Rezdiffra.
The new MASH indication adds to earlier approvals for the drug in weight management and cardiovascular risk reduction in people with overweight or obesity.
Novo Nordisk was also developing FGF21 drug zalfermin as a MASH treatment, but discontinued the programme after concluding that it did not show significant differentiation from semaglutide in efficacy.












