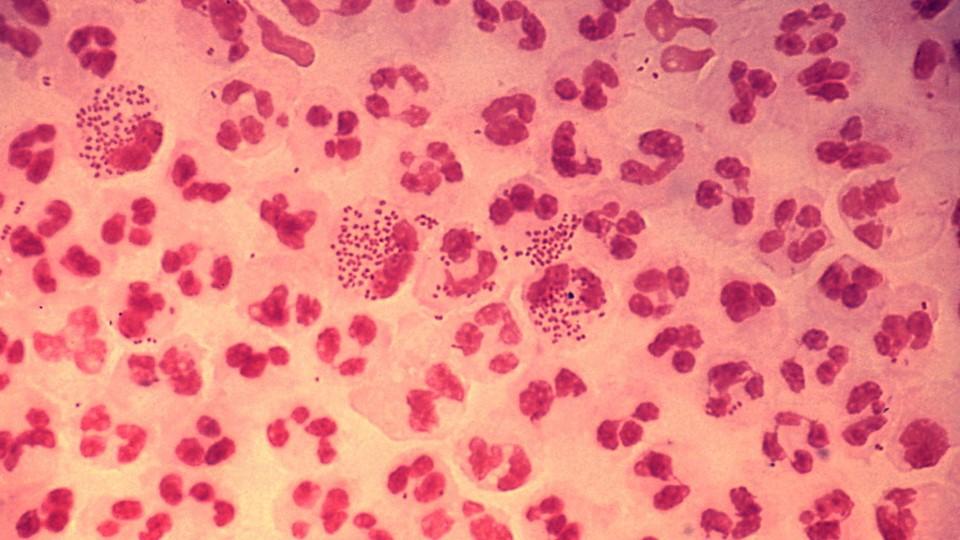
FDA starts swift review of GSK antibiotic for gonorrhoea
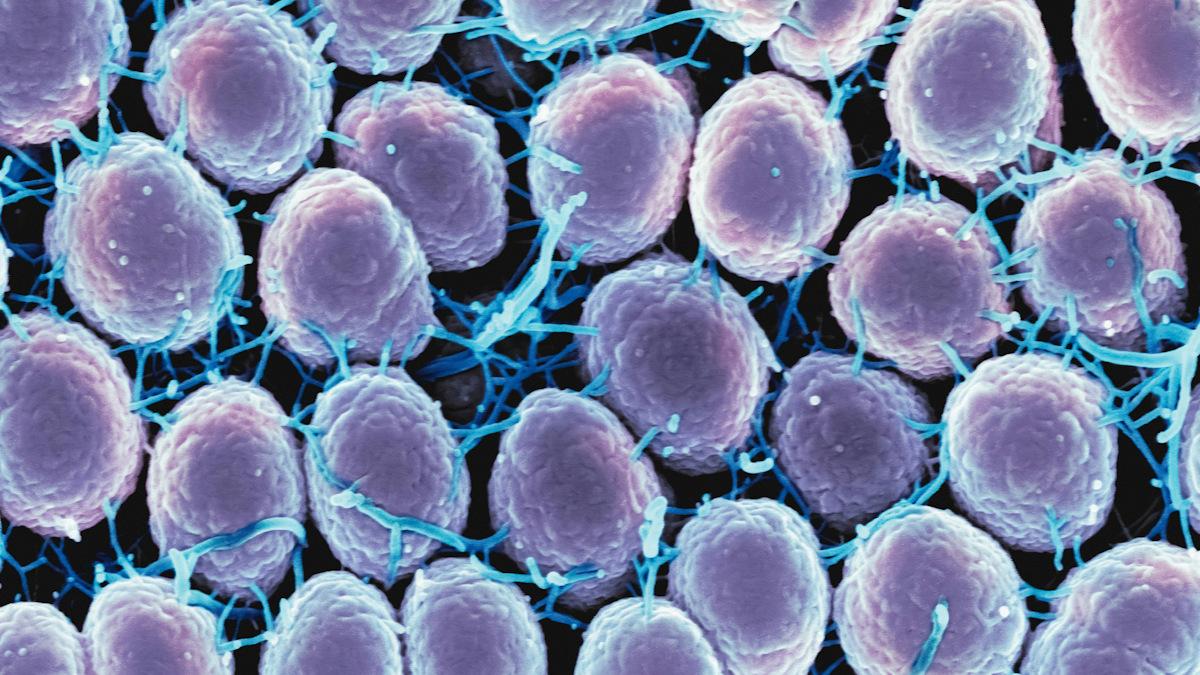
The FDA has started a priority review of GSK's oral antibiotic gepotidacin as a treatment for uncomplicated gonorrhoea, with a decision due before the end of the year.
If approved, gepotidacin would offer an oral option to many US patients who currently have to rely on injectable treatments for the sexually transmitted infection (STI), caused by the Neisseria gonorrhoeae bacteria, which is classed as an urgent public health threat by the CDC.
There is a pressing need for new treatment options for uncomplicated gonorrhoea infections, which have a high level of morbidity, enhance the transmission of other STIs, and are highly stigmatised, often causing shame and affecting personal relationships. Without effective treatment, gonorrhoea can lead to infertility and other sexual and reproductive health complications like pelvic inflammatory disease.
There were more than 600,000 cases of gonorrhoea reported in the US in 2023, according to the CDC, making it the second most commonly reported STI.
Another novel oral antibiotic, Innoviva's zoliflodacin, is also undergoing FDA review for uncomplicated gonorrhoea after being filed for approval in June.
GSK has filed gepotidacin on the strength of the EAGLE-1 trial, in which two oral doses of the antibiotic proved to be just as effective as standard combination therapy with intramuscular ceftriaxone plus oral azithromycin in resolving uncomplicated N gonorrhoeae infections.
Gepotidacin was non-inferior to ceftriaxone/azithromycin at achieving a microbiological response at the 'test-of-cure' clinical visit, which takes place between three and seven days after treatment starts. There were also failures at the urogenital site due to bacterial persistence of N gonorrhoeae at either dose tested.
Gepotidacin was approved by the FDA earlier this year, under the Blujepa brand, as the first oral antibiotic to offer a new mechanism of action in uncomplicated urinary tract infections (uUTIs) in nearly 30 years. That indication was also granted a priority review by the agency, and is also under review in the UK and Australia.
The drug inhibits DNA replication via two different bacterial type II topoisomerase enzymes, and its dual mode of action should – at least in principle – make it harder for pathogens to develop resistance. Zoliflodacin also works by targeting type II topoisomerases.
Gonorrhoea ranks high among the World Health Organization's infectious diseases of greatest concern, as it is rapidly becoming resistant to antimicrobial medicines.
Photo by National Institute of Allergy and Infectious Diseases on Unsplash