AZ plots wider Imfinzi use in bladder cancer after trial win
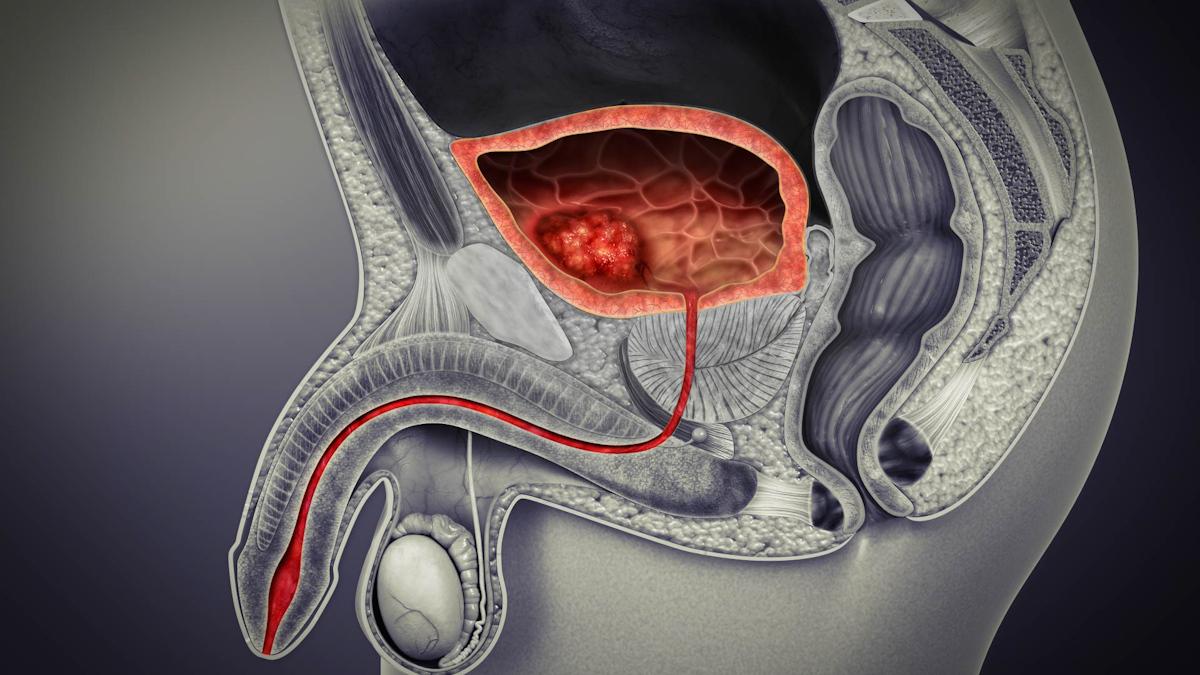
AstraZeneca's run of positive trial results with its cancer therapies has continued with a win for its immunotherapy Imfinzi in non-muscle invasive bladder cancer (NMIBC).
Top-line results from the POTOMAC trial revealed this morning show that one year of treatment with PD-L1 inhibitor Imfinzi (durvalumab) plus standard BCG induction and maintenance therapy achieved a "statistically significant and clinically meaningful improvement in disease-free survival (DFS)" compared to standard treatment alone.
A second arm of the study comparing Imfinzi plus BCG induction-only therapy to BCG induction and maintenance therapy alone did not reach statistical significance for DFS.
The new data comes shortly after Imfinzi was approved as a peri-operative treatment for muscle-invasive bladder cancer (MIBC), based on the results of the NIAGARA trial, which marked a return to the bladder cancer area.
The drug claimed accelerated approval in previously treated adult patients with locally advanced or metastatic forms of the disease in 2017, although that was subsequently withdrawn after the failure of a confirmatory study.
The new NMIBC indication, which is being prepared for filing, represents a "meaningful new opportunity" for Imfinzi, which brought in around $4.7 billion in sales for AZ last year from its current labelling across lung, liver, endometrial, biliary tract, and bladder cancers, according to recent comments by AZ's head of oncology R&D, Susan Galbraith.
That said, there has been a string of recent approvals in NMIBC that have made the segment more competitive, including for MSD's rival PD-1 inhibitor Keytruda (pembrolizumab), ImmunityBio's Anktiva, and Ferring's Adstiladrin, while Johnson & Johnson is expecting a verdict from the US FDA later this year for its TAR-200 candidate.
More than 70% of bladder cancer patients are diagnosed with NMIBC, an early-stage cancer where the tumour is in the tissue that lines the inner surface of the bladder but has not invaded the muscle wall, according to AZ. Among these, around half will be at high risk of progression to more serious disease.
POTOMAC's lead investigator, Maria De Santis of Charité Universitätsmedizin Berlin in Germany, said that the DFS results are "exciting" because "while most patients with [NMIBC] are treated with curative intent, 80% see their disease return and almost half may require life-altering surgery to remove the bladder."
Imfinzi is being tested in a range of studies across early and late-stage bladder cancer in various treatment combinations, including the VOLGA study in patients with MIBC who are ineligible or refuse to take cisplatin and the NILE study as a first-line therapy versus chemotherapy in locally advanced or metastatic disease.
The new result follows hard on the heels of positive data with Daiichi Sankyo-partnered Enhertu (trastuzumab deruxtecan) in early-stage and advanced forms of breast cancer.
Image by scientificanimations.com via Wikipedia












