Mixed reactions among HCPs as lecanemab and donanemab secure FDA approval for Alzheimer's treatment

Last month, the FDA granted approval to Eli Lilly’s donanemab - or Kisunla - for the treatment of Alzheimer’s disease. Its approval was based on the treatment demonstrating what the FDA described as a “statistically significant reduction in clinical decline on the integrated Alzheimer's Disease Rating Scale (iADRS) compared to placebo”. Anne White, Eli Lilly’s executive vice president, said that the amyloid plaque-targeting therapy had shown “very meaningful results for people with early symptomatic Alzheimer's disease.”
Donanemab’s approval was the latest development in what many have seen as two years of significant developments in the Alzheimer’s treatment space, with Lilly’s drug not the only monoclonal antibody treatment that has entered - and is shaping - the landscape of Alzheimer’s care. Its approval succeeds both the FDA’s accelerated and traditional approvals of Eisai and Biogen’s lecanemab (also known as Leqembi), a similar treatment, in early and mid-2023 respectively.
Among healthcare professionals (HCPs) themselves, though, research has found a mixed bag of perspectives. While some have praised the “wonderful news” as a “real success” for Alzheimer’s treatment, others have been considerably more cautious. One described the approval of lecanemab as “no panacea” for patients, for example, while another went as far as to suggest that, in their eyes, donanemab offered “no net benefit” at all.
Listening to the social media conversations of healthcare professionals affords an opportunity to understand precisely what is fuelling this divergence in perspective. This article will explore exactly what HCPs had to say as these two key treatments navigated their way through the regulatory process.
Lecanemab
In January 2023, lecanemab received accelerated approval for the management of mild cognitive impairment associated with Alzheimer’s, based on its ability to reduce amyloid plaques in patients - a key pathological feature of the disease.
Mainstream health outlet WebMD described the treatment’s approval as “offering hope” to patients where there has in the past “been little” - providing a novel mechanism to slow the progression of Alzheimer’s.
Analysing the social media conversations of healthcare professionals immediately after the approval revealed mixed responses, however. Some were positive, but overall sentiment was tempered by calls for more comprehensive data to fully assess the drug’s efficacy and safety profile. There were particular concerns about the risks of amyloid-related imaging abnormalities and cerebral haemorrhage.
Perhaps most emblematic of this overarching sentiment was a post by Miles Cobia, an Alabama-based neurologist, who said he was “cautiously optimistic” about the treatment's potential, conceding however that there were “still many answers needed”.
Similar was the case for Mikkael A. Sekeres MD, MS, who noted that, while lecanemab did show some signs of slowing Alzheimer’s progression, the drug was “no panacea” for patients, given its relative impact versus placebo. Neurologist Madhav Thambisetty emphasised the “urgent need” for “greater clarity to determine whether the findings represent a meaningful advance” in attempts to tackle dementia.
Physician Jessamy Bagenall shared a quotation from a StatNews round-up which, she said, illustrated the “difficulty for clinicians in prescribing [a] drug with uncertain benefits for patients often desperate for something.”
In July 2023, lecanemab received a full traditional FDA approval, cementing its role in the treatment landscape. Broader Medicare coverage for patients followed, which was celebrated by several HCPs.
Julien Cavanagh, MD, in response to the approval, posted that “even though modest, the improvement awarded by lecanemab in Alzheimer’s disease is a breakthrough.”
That sense of hesitation as to the transformative potential of the treatment was echoed in other HCP posts, too. Esther Choo, MD MPH, for example, shared an article in which she wrote: “Does that mean that lecanemab is a game changer? No, not yet.”
HCP conversations also centred on pricing, highlighting the treatments' comparative affordability against earlier therapies.
Donanemab
In May of 2023, two months prior to the full approval of lecanemab, another promising treatment for the disease, Eli Lilly’s donanemab, emerged from Phase III trials. It reported data that demonstrated, according to a Lilly press release, “significantly slowed cognitive and functional decline in people with early symptomatic Alzheimer's disease.”
Online conversations among HCPs exhibited mixed reactions to this news, echoing the cautious optimism that accompanied discussions about lecanemab earlier in the year.
Some HCPs were especially positive, though. Psychologist Matt Wall, for example, praised the “incredible news” of the treatment's trial success: “[It] feels like we might be getting somewhere with these treatments.” Andrew Stern, MD, PhD, offered similar thoughts, celebrating the “wonderful news for patients and families” and hoping that the results were “just the first steps toward a slow and steady improvement in outcomes.”
Others described the results as “really exciting news” for patients, and a “wonderful result for the field”.
Hesitancy nonetheless lingered. Christin Glorioso, MD PhD, said that there was “still much to improve in terms of safety and efficacy”. Another HCP queried whether donanemab would be any safer than lecanemab and its associated risks, considering their similar efficacies.
When the trial data was presented in full at the Alzheimer's Association International Conference (AAIC) 2023, in July, the same sentiment stood firm. A post by Dr Richard Simpson reflected this. While he said that there was indeed “room for optimism and avenues to explore”, he conceded that “those hoping for a magic bullet may be sorely disappointed.”
By the time that the FDA finally approved donanemab for Alzheimer’s patients in July of this year, our research found that HCP engagement was markedly lower than the major developments and trial results of 2023. Much of this activity centred on general news sharing posts from HCPs.
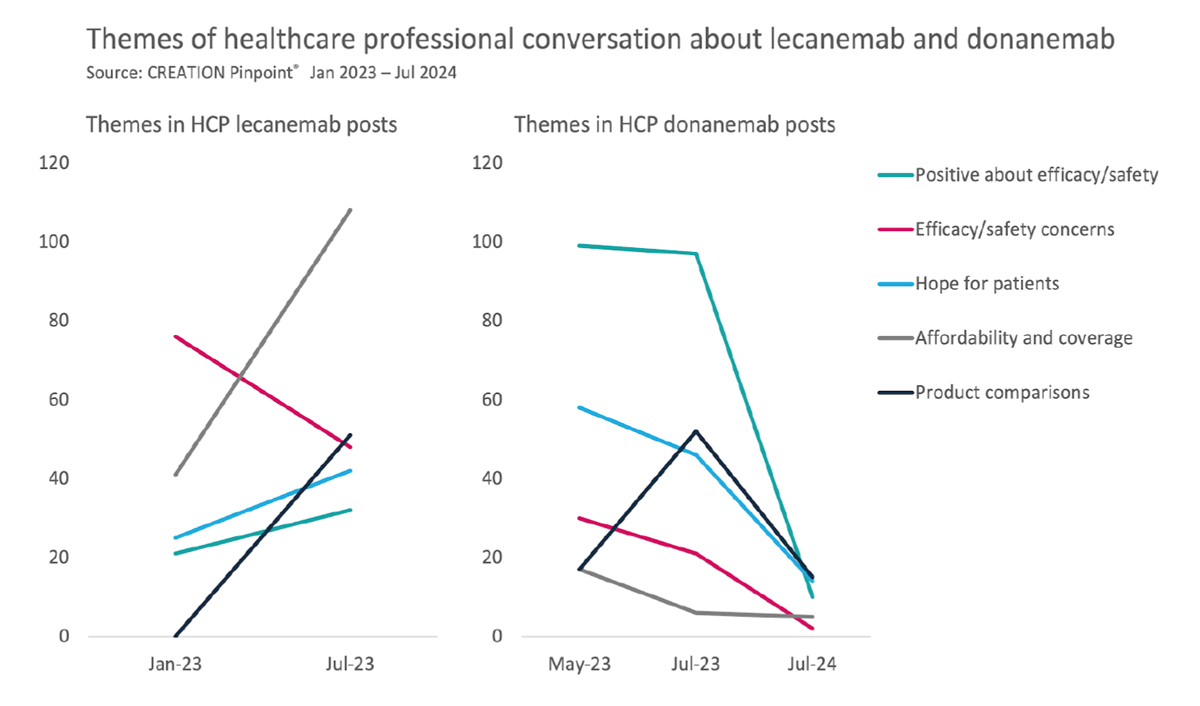
There were, nonetheless, a small number that took to social media to share their thoughts. One of these was neuroradiologist Suzie Bash, who described the approval as offering “exciting new options” for patients. Several HCPs also discussed the approval alongside information about the side effects associated with donanemab. One HCP appeared critical of the FDA decision to approve what he saw as a “high-risk, low-reward” treatment.
Overall, there were approximately half as many posts from HCPs responding to donanemab’s approval compared to that of lecanemab. Very few of these expressed an opinion. The themes that had previously been so prominent around efficacy, safety, affordability, and head-to-head comparisons all declined, some quite dramatically.
A broader consensus around lecanemab and donanemab for now appears elusive. The European Medicines Agency recently rejected a license for the former, on the basis that “the benefits of treatment are not large enough to outweigh the risks.”
Listening to and engaging with the online conversations of healthcare professionals provides a unique and valuable opportunity to identify and understand how these debates are unfolding beyond the upper echelons of the pharmaceutical industry, and instead among those on the frontlines of delivering these treatments to patients across the world.












