How integrated patient experience platforms are revolutionising data capture

Clinical trials are currently experiencing a quiet renaissance that is fundamentally changing the way medical research captures and quantifies patient experience data. As regulators increasingly advocate for patient-focused drug development, the importance of high-quality patient experience data has never been higher.
And it’s here that advances in electronic clinical outcome assessment (eCOA) platforms and digital health technologies are driving the collection of robust, traceable, and authenticated patient experience data.
As the demands on technology increase, interoperability between medical devices and digital health platforms is essential. It eliminates manual data entry, which has plagued clinical research for decades, and simplifies patient routines while improving data insights and fidelity through real-time health monitoring and AI-powered insights.
Obesity as a mega-therapeutic area
Nowhere is this integration more pronounced than in obesity research. In the rapidly growing therapeutics market, Morgan Stanley Research “estimates the global market for obesity drugs could reach $150 billion at its peak in 2035, an increase from a previous forecast of $105 billion. In 2024, this market had about $15 billion in sales.” Obesity is on course to join oncology, antidiabetics, and immunology as a mega-therapeutic area.
As of June 2025, there were 173 obesity drugs in development or being marketed with multiple mechanisms beyond the leading GLP-1 therapies. That’s 53 more than were being studied just a year prior. The expanding range of therapeutic targets and mechanisms is creating both a complex research landscape and notable clinical progress.
Obesity treatments vary in their objectives, with some drugs designed to achieve weight loss and others to sustain weight maintenance. The net outcome is increased pressure to conduct more streamlined, patient-centric trials.
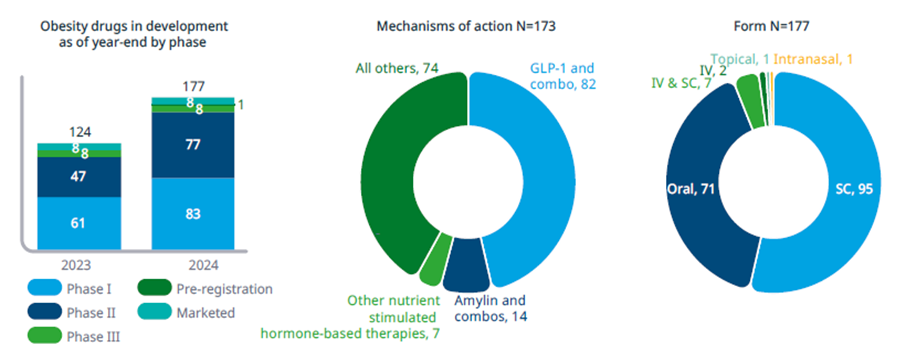
Figure 1: 173 Obesity Medicines in Development. Source: Citeline Trialtrove (January 2025); IQVIA Institute (January 2025)
Why integration is crucial now
Several converging trends make this a particularly critical moment for clinical trial technology:
- Market pressure: The therapeutics boom in obesity has created historic demand for streamlined trials that can handle long-term patient engagement. Obesity trials frequently run for years and require ongoing measurement of multiple endpoints, so minimised patient burden is a prime driver of trial success.
- Technology maturity:Technology has reached a level of maturity that allows integration of various systems and devices to be seamless. Connected health devices and cloud platforms have evolved from simple data capture tools to sophisticated systems capable of real-time data synchronisation and workflow automation.
- Patient expectations: Post-pandemic, patients desire more digital-first healthcare experiences, such as telehealth, remote monitoring, apps, or patient portals. In tandem with this, regulatory bodies have evolved to facilitate digital innovations.
- Regulatory evolution:The FDA’s emphasis on patient-centred drug development is driving broader adoption of patient-reported outcomes gathered on a range of technology platforms.
Trial complexity, such as that of obesity trials, exacerbates the need for integrated technologies. As shown in Figure 2, patients with obesity often experience multiple co-morbidities: 59% experience cardiovascular disease or hypertension, 56% experience dyslipidemia, 33% experience degenerative joint disease or osteoarthritis, and 32% experience type 2 diabetes or metabolic syndrome. These varying co-morbidities demand that trials measure a wide variety of endpoints ranging from glucose levels and body composition to quality-of-life surveys and physical activity measurements. Capturing this data across fragmented systems places additional burden on patients and introduces multiple points of potential error.
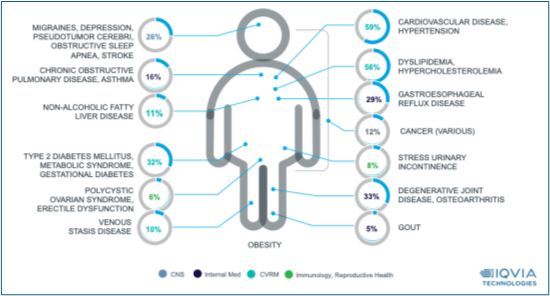
Figure 2: Prevalence of common co-morbidities based on US open claims data
The impact of innovation
Traditional clinical trials require patients to manually report device readings, complete separate questionnaires, and transcribe data, which, as noted above, potentially introduces multiple points of failure and adds significant patient burden. Integrated eCOA platforms fundamentally revise this model by passively capturing device data, triggering contextual questionnaires in the moment and creating unified datasets without manual intervention.
Consider the case of obesity research using blood glucose meters. In “classic” trial modality, when a patient experiences a hypoglycaemic episode, they need to read their glucose meter, record the reading on paper or in another diary and then complete a questionnaire about symptoms during the episode. By that point, memory might have faded and valuable information may be lost, or they may forget to record the episode altogether.
With an integrated system, the glucose meter automatically logs when blood sugar dips below a threshold. This automatically creates a notification on the patient’s smart device prompting them to complete a brief symptom questionnaire as soon as physically able. Further reminder notifications can be triggered to drive near-real-time data collection to minimise recall bias. The glucose level is automatically linked to the questionnaire responses, creating a complete, time-stamped record of both the objective measure and the subjective patient experience. This captures critical data that would likely otherwise be lost during disorienting low-blood-sugar incidents.
The technical infrastructure supporting this integration involves several key components. API integrations between medical devices and eCOA platforms allow data to flow transparently between systems. Real-time data sync protocols make data available immediately instead of being batched and processed later. Cloud-based data lakes enable multi-stream integration by bringing together device data, patient-reported outcomes, site-based assessment, and other sources into a single source of truth. In addition, security frameworks offer HIPAA-compliant device connection to protect patient data while making it accessible to authorised users.
Beyond data collection with advanced applications
The real promise of integrated platforms is more than simply eliminating manual data entry. For example, they allow entirely new trial design and patient monitoring approaches. Machine learning algorithms can data-mine integrated patterns to predict patient compliance and identify patients at risk for dropping out of a trial and/or requiring additional support, and adaptive trial designs can modify procedures based on real-time findings, making studies more efficient and responsive to safety signals arising during the study.
AI-powered patient engagement systems can be triggered by device data to provide personalised support at the moment of need. For instance, when a patient’s activity sensor detects declining physical activity levels – a common early indicator of disengagement – the system can automatically provide motivation or notify study coordinators to initiate contact. This proactive approach to compliance management can significantly improve trial retention rates over the long term.
Integration also enables more sophisticated safety monitoring. When glucose levels, blood pressure, weight, and patient-reported symptoms all flow into a single system with synchronised time stamps, researchers can identify correlations and patterns that would be hidden in data silos. An adverse event that would be minor in isolation may reveal itself to be part of a concerning pattern when viewed in the context of multiple streams of data.
Benefits across stakeholders
The utility of integrated platforms is passed on to every stakeholder within the clinical trial ecosystem. For patients, automation and passive data capture substantially reduce burden. Instead of spending time manually processing readings and completing several different tasks, patients can focus on their health while technology works in the background. This transforms the trial experience from burden to empowerment. This can be particularly important for obese patients who have often experienced multiple treatment failures and who may view trial participation both as access to novel treatment and as a way of contributing to scientific advancement.
For sites, integrated systems provide richer data insights and safety monitoring. Real-time dashboards give site staff immediate insight into patient compliance, safety concerns, and data quality issues. Automated mapping of device readings to diary entries slashes data reconciliation efforts exponentially, freeing up site staff time for hands-on patient care.
Trial sponsors benefit from an integrated view of patient data and expedited timelines. When all data streams are combined in real-time instead of being reconciled months later when the database is locked, trial teams can identify and fix problems much sooner. Months can be cut from development timelines, which can equate to tangible operational advantages in highly competitive therapeutic categories like obesity, where multiple companies are racing to bring new therapies to market.
For the broader industry and scientific community, integrated platforms produce new insights into how to structure future research. The integrated, time-synchronised data they generate enables new types of analysis that render transparent relationships between variables that were previously obscured by data silos and time lags.
The road ahead
Looking to the future, AI-powered integrated platforms offer an elemental shift in the way that clinical trials are conducted, with the focus changing from simply collecting data to understanding the complete patient experience in context.
Those who make the shift successfully will have a significant advantage in bringing novel treatments to market. But beyond competitive advantage, thoughtful technology design can move the patient experience from burden to empowerment. For patients participating in groundbreaking research, seamless and dignified data capture is not simply a matter of contributing data points, but of advancing science without compromising quality of life. Holistic patient experience platforms aren’t just streamlining efficiency, they’re positively changing what’s possible in medical research, one automated data point at a time.
About the author
 Melissa Mooney is director of the Digital Patient Suite at IQVIA. She has more than 20 years of experience in the development of eCOA solutions for use in clinical trials. Mooney’s area of expertise is eCOA solution design, where she has helped clients and eCOA vendors to develop robust and usable eCOA software solutions that meet eCOA protocol requirements. She also brings a plethora of experience in eCOA requirement gathering, leading eCOA user acceptance testing, eCOA data management, and business development support.
Melissa Mooney is director of the Digital Patient Suite at IQVIA. She has more than 20 years of experience in the development of eCOA solutions for use in clinical trials. Mooney’s area of expertise is eCOA solution design, where she has helped clients and eCOA vendors to develop robust and usable eCOA software solutions that meet eCOA protocol requirements. She also brings a plethora of experience in eCOA requirement gathering, leading eCOA user acceptance testing, eCOA data management, and business development support.












