The evolution of real-world evidence
Once on the sidelines of scientific decision-making, real-world evidence has steadily taken centre stage – reshaping how the value of medicines is measured, demonstrated, and delivered. Powered by digital transformation, policy shifts, and a growing demand for data that reflects real patients in real settings, RWE has evolved from a quiet companion to clinical trials into a leading actor in the story of market access.
Here, we trace RWE's remarkable evolution from its handwritten roots through technological breakthroughs, methodological advancements, and regulatory milestones that have collectively elevated real-world evidence from peripheral consideration to central pillar in pharma market access.
Pre-1900s:
Data by candlelight
Long before the days of digital health records or sophisticated analytics, the foundational principles of real-world evidence were established by pioneering medical researchers, such as Dr Ignaz Semmelweis and Florence Nightingale, who in mountains of meticulously handwritten notes captured their observations of health outside of the carefully controlled experiment environment.
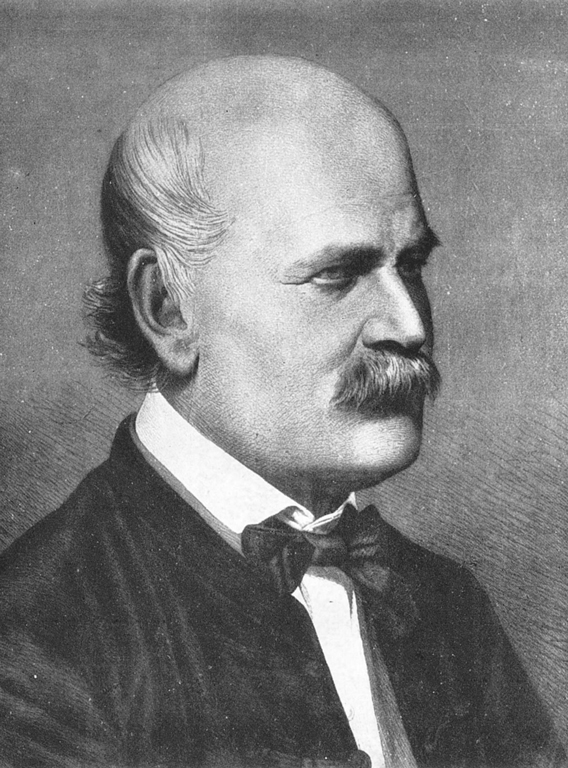
Pictured: Ignaz Semmelweis c. 1860. Image credit: Public domain, via Wikimedia Commons
In the 1840s, renowned "saviour of mothers" Semmelweis recognised a tragic phenomenon occurring within Vienna General Hospital's maternity clinics. The clinic was split into two departments, one operated exclusively by midwives, the other by trained clinicians and medical students. Yet, Semmelweis noted a much higher mortality rate of mothers being treated in the doctor-run department. Five times higher. To find out why, he began to gather statistical evidence, which ultimately revealed that hand washing dramatically reduced maternal deaths from postpartum infection. While his research was met with ire from the bourgeoisie medical establishment, Semmelweis's systematic real-world observations proved correct, representing one of the earliest examples of using systematic real-world observations to improve clinical practice.
-768px.jpg)
Pictured: Florence Nightingale. Image credit: Henry Hering (1814-1893), public domain, via Wikimedia Commons
Similarly influential was Florence Nightingale's groundbreaking work during the Crimean War (1853-1856). Seeking ways to improve the horrific treatment conditions for wounded soldiers, she turned to an underused asset at the time – data. Using innovative statistical visualisation – including her famous "rose diagrams" – she was able to demonstrate that poor sanitary conditions, not battle wounds, were the primary killer of soldiers.
These early pioneers established the critical principle that systematically collected observational data from actual practice settings could reveal insights impossible to obtain through other means. Throughout the early and mid-20th century, epidemiological studies and disease registries further developed these methodologies, gradually building toward the more formalised approaches to real-world evidence that would emerge in the decades later.
1960s-1980s:
Dawn of (costly) electronic health records
As cultural revolutions swept the streets, the 1960s also gave rise to a different type of transformation, one that would form a critical piece of modern real-world evidence: electronic health records. In 1961, IBM partnered with Akron Children's Hospital to implement one of the earliest experimental electronic systems. Though primitive by today's standards, the process saw healthcare staff enter patient-provided information into the rather hefty IBM Ramac 305 computer, which looked more like a kitchen set-up than today's slimline laptops.
-768px.jpg)
Pictured: IBM 305 main system (processing unit, magnetic process drum, magnetic core register, electronic logical and arithmetic circuits), Image credit: Norsk Teknisk Museum, CC BY-SA 4.0, via Wikimedia Commons
By the late 1960s, Dr Lawrence L Weed developed what many consider the first true electronic medical record, dubbed the Problem-Oriented Medical Information System (PROMIS), while working at the University of Vermont. Unlike earlier systems, which focused primarily on billing, PROMIS was designed to improve clinical care by organising patient information around medical problems, rather than prioritising provider convenience.
Another significant breakthrough came in 1972, when the Regenstrief Institute in Indianapolis developed a more comprehensive electronic medical record system. Led by Dr Clement McDonald, this ambitious project aimed to solve the fundamental challenge of fragmented patient information across different providers and settings. Working with computer scientists from Purdue University, McDonald's team created new database architecture that would later influence modern health information systems. Though initial adoption was limited by enormous costs and technological constraints, this system evolved dramatically – eventually growing from 35 diabetes patients in 1972 to housing nearly 300 million separate results for over 3 million patients, decades later.
Throughout the 1970s and early 1980s, the colossal sum required to establish and operate the tools required to capture data meant that only major academic medical centres and research institutions could afford to implement electronic health records. The majority of institutions, both large and small, were written or typed on paper, which meant that health records had to be stored and moved manually.
1990s:
Building the infrastructure for RWE
The widespread adoption of home computers throughout the 90s saw their popularity soar in smaller clinics and private practices, meaning that more and more people had access to advanced data collection and communication methods. As such, the decade marked the nascent period for what would eventually become known as real-world evidence in pharma. During this time, the term "outcomes research" first entered the industry lexicon, and in 1995, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), now known as ISPOR, was formally established.
Pharmaceutical companies began conducting retrospective analyses using insurance claims databases, primarily for post-marketing surveillance, rather than to support pricing or reimbursement decisions. For rare diseases and specialised treatments, patient registries emerged as valuable data collection tools, though these efforts were often fragmented and lacked standardisation. Ever cautious, regulatory agencies and payers maintained scepticism toward observational data being used to support market authorisation, firmly prioritising randomised controlled trials as the gold standard for evidence.
2000-2010:
Regulatory recognition and strategic adoption
Having survived the dreaded "Y2K bug", the turn of the millennium saw significant maturation in real-world data appreciation, as pharma companies established dedicated Health Economics and Outcomes Research (HEOR) departments, designed to systematically capture and analyse post-approval drug performance.
Early patient-reported outcome measures also emerged during this period, acknowledging that patient experiences constituted a vital dimension of drug value previously uncaptured in traditional efficacy measures. The accelerating adoption of electronic health records created unprecedented access to longitudinal patient data, though interoperability challenges limited full utilisation of these resources.
In 2004, the FDA launched its ambitious Sentinel Initiative, signalling regulatory recognition of real-world data's importance in monitoring drug safety. That same year, leading open source enterprise electronic medical record system platform, OpenMRS was launched in Eldoret, Kenya. Led by Regenstrief Institute and Partners In Health, the multi-institution, non-profit collaborative was initially launched to help manage patient information at AMPATH healthcare facilities as part of efforts to combat the HIV/AIDS outbreak. It is now available in more than 80 countries, serving over 22 million patients.
Four years later, in 2008, a public-private partnership involving the FDA, multiple pharmaceutical companies, and healthcare providers known as the Observational Medical Outcomes Partnership (OMOP) was launched to develop methods and tools for analysing observational healthcare data. Over its five-year lifespan, OMOP developed methodologies, tools, and shared resources for analysing diverse healthcare data sources, successfully establishing a common informatics infrastructure that could handle both claims and electronic health records from around the world. OMOP's work in standardising data models would prove pivotal in demonstrating the feasibility of large-scale statistical analyses for active drug safety surveillance across prescription medications.
Introduction of the HITECH Act in 2009 continued to build on the momentum RWE had gained throughout the decade, significantly accelerating electronic medical record adoption across healthcare systems and expanding the potential data pool for real-world studies. Meanwhile, across the Atlantic, European Health Technology Assessment (HTA) bodies cautiously began to accept supplementary real-world evidence in select submission packages, primarily to address uncertainty around long-term outcomes.
2010-2020:
Integration, acceleration, and sophistication
Regulatory acceptance of real-world evidence reached a critical tipping point during the early 2010s, with multiple authorities establishing formal pathways for its consideration. The FDA's Sentinel System became fully operational in 2014, creating a sophisticated, active surveillance system leveraging real-world data from multiple sources to monitor drug safety. That same year, the European Medicines Agency launched its adaptive pathways pilot programme, explicitly incorporating real-world evidence collection into regulatory decision-making for selected products.
The UK's National Institute for Health and Care Excellence (NICE) began accepting certain categories of real-world evidence in its evaluation processes, particularly for treatments with significant uncertainty. The pharmaceutical industry responded by investing in specialised RWE platforms and sophisticated analytics capabilities, recognising the strategic importance of generating compelling real-world data. Patient advocacy groups increasingly pushed for real-world performance metrics that better reflected outcomes meaningful to patients' daily lives. Perhaps most significantly, risk-sharing agreements between manufacturers and payers emerged, directly tying reimbursement to real-world performance metrics, fundamentally altering the market access landscape by creating mechanisms where payment aligned with actual patient outcomes, rather than promised benefits.
The landmark 21st Century Cures Act of 2016 formalised RWE's role by mandating the FDA consider real-world evidence in certain approval decisions, signalling a paradigm shift in regulatory thinking. The European Medicines Agency's PRIME programme similarly incorporated real-world evidence considerations for priority medicines. The FDA subsequently developed a comprehensive framework for its RWE Program in 2018, providing clear guidance on evidence standards and appropriate applications.
Perhaps most notably, in 2018, Pfizer's Ibrance (palbociclib) became the first oncology therapy to receive FDA label expansion based primarily on RWE – specifically, data derived from Flatiron Health's EHR database. In parallel, the US FDA published a framework for the organisation's real-world evidence programme, while in 2019 the European Union introduced the OPTIMAL framework for Real-World Evidence (RWE). This framework, based on three pillars – operational, technical, and methodological –was designed to ensure the proper use of RWE in the EU's drug regulation process.
2020-Present:
From experimental to essential
The COVID-19 pandemic was a catalyst for mainstreaming RWE in market access discussions. During the crisis, real-world data proved critical in assessing the effectiveness and safety of vaccines and treatments in real-time, outside the limitations of clinical trial environments. The urgency to generate evidence at speed accelerated regulatory and payer comfort with alternative data sources. However, issues around data quality, standardisation, and general availability remained prominent, and calls quickly began to improve the accuracy of data across healthcare systems. At the same time, artificial intelligence and machine learning applications emerged to extract insights from increasingly complex and voluminous real-world datasets.
By 2021, the EMA issued draft guidance to formalise the role of RWE in regulatory decision-making across member states, further aligning Europe with US advancements. One year later, in 2022, the UK's National Institute for Health and Care Excellence (NICE) released its much-anticipated real-world evidence framework, providing guidance on conducting RWE studies, assessing data suitability, and methods for real-world studies of comparative effects.
NICE and the MHRA deepened their collaboration in 2023 by forming a joint advisory group on RWE, aimed at streamlining evidence standards and enabling greater consistency in regulatory and market access use cases. Across markets, RWE is now a strategic asset in payer negotiations, often used to support outcomes-based agreements and managed access schemes.
Recent years have witnessed remarkable integration of real-world evidence throughout drug development and commercialisation processes. Synthetic control arms incorporating RWE have begun supplementing or partially replacing traditional control groups in clinical trials, particularly for rare diseases or conditions with established standard therapies. Regulatory agencies have demonstrated increasing comfort, approving label expansions and new indications based primarily on robust real-world evidence.
As we move through the mid-2020s, the role of RWE in pricing, reimbursement, and post-launch evidence generation continues to expand. Market access teams are increasingly expected to provide real-world validation of therapeutic value to secure optimal formulary positioning. The launch of EMA's DARWIN EU platform – a centralised network for generating real-world evidence across European data partners – represents a major leap toward scaled, structured use of RWE at the regulatory level, however, major political shifts in the US threaten the stability of this data ecosystem.
Pharmaceutical companies are also embedding RWE earlier in the product lifecycle, using it to inform clinical trial design, patient segmentation, and health economic modelling. With global stakeholders – from regulators and HTA bodies to payers and providers – now aligned on the importance of evidence beyond the trial, real-world data is no longer a supplement to market access strategy; it's a cornerstone.
About the Author
Eloise McLennan is the editor for pharmaphorum’s Deep Dive magazine. She has been a journalist and editor in the healthcare field for more than five years and has worked at several leading publications in the UK.
Supercharge your pharma insights: Sign up to pharmaphorum's newsletter for daily updates, weekly roundups, and in-depth analysis across all industry sectors.
Want to go deeper?
Continue your journey with these related reads from across pharmaphorum
Click on either of the images below for more articles from this edition of Deep Dive: Market Access and Commercialisation 2025