A problem combination?

Leela Barham takes stock of past NICE decisions for combinations to explore the scale of the reimbursement challenge for combination therapies.
Combination therapies are now common in therapy areas like cancer. There have been cases where even if a new treatment used in combination were offered at zero cost to the NHS that it would not be cost-effective despite being clinically effective. A situation described by researchers as illogical. But how big a problem is it?
NICE statistics
NICE has worked hard at improving the transparency of their work and that includes providing a downloadable excel spreadsheet of all their Technology Appraisal (TA) recommendations.
By reviewing the name of the technology appraised, it’s possible to identify when NICE has looked at a combination (this assumes that NICE consistently uses the term ‘combination’ in the name of the technology, if they don’t some combinations will have been missed).
Since 2000/01 and based upon the excel downloaded on the 10 August 2021, NICE has made 127 recommendations for combination therapies. That’s 13 per cent of 942 recommendations NICE has made for pharmaceuticals.
Combinations vs non-combination therapies
Whilst there are now well known case studies for when NICE has not recommended a combination – researchers have cited pertuzumab in combination with trastuzumab and docetaxel for breast cancer, vinflunine for advanced or metastatic transitional cell carcinoma and cetuximab for head and neck cancer - just how often does NICE not recommend a combination?
It turns out that close to 1 in 4 NICE recommendations for a combination therapy is negative. That compares to more like 1 in 10 NICE recommendations when NICE has looked at non-combinations (Figure 1).
Figure 1: NICE Technology Appraisal recommendations for combinations and non-combinations
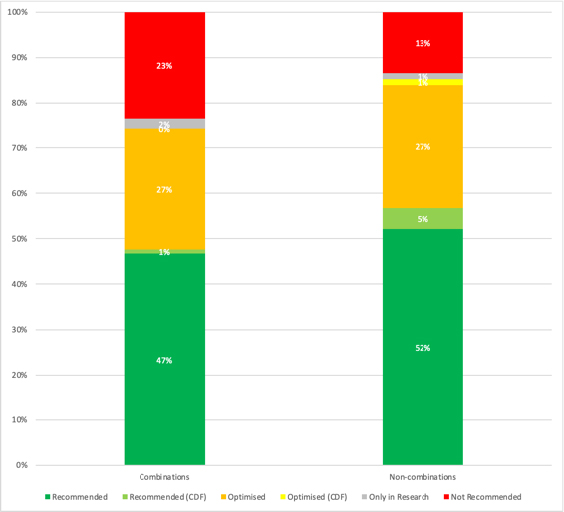
Source: Analysis of NICE data. Note 2021/22 data is data available up to 10 August 2021 and is therefore not a full financial year of data. Excludes Terminated Appraisals due to non-submissions. 124 recommendations for combinations, 746 recommendations for non-combinations.
Perhaps surprising is that non-combinations are more likely to have non-submissions than combinations. You might have expected more; this is because companies will be aware that it is difficult to achieve a positive NICE recommendation given that combinations are often high cost, especially when the backbone therapy is still on patent. But in any case, non-submissions are very much a recent phenomenon for both combinations and non-combinations (Figure 2).
Figure 2: NICE Technology Appraisal Terminated Appraisals – non-submissions for combinations and non-combinations, 2000/1 to 2021/22
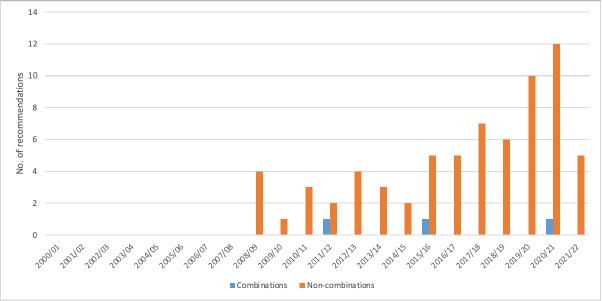
Source: Analysis of NICE data. Note 2021/22 data is data available up to 10 August 2021 and is therefore not a full financial year of data.
A need for a closer look
This analysis suggests that there is a higher chance of NICE saying no to a combination. But what it has not addressed is why and is whether there is a bigger issue in some therapy areas more than others, nor looked at the detail of the combinations. Analysis of cancer combinations is an obvious contender for further work given that this is where the examples cited by researchers come from.
It is fair to say some combinations will not present such a reimbursement challenge because they are combinations of a new treatment with one that is off patent. For example, NICE has made recommendations or combinations that include aspirin. The bigger problem will be those combinations of on-patent treatments used together, assuming that price is the biggest driver of the negative NICE recommendation.
Future for combinations
But is it a problem that NICE says no more often to combinations? It could be if there are therapies that are clinically important that are not being made available and where there could be some room for manoeuvre on cost.
The pricing challenge can be a tough nut to crack when there are different companies involved for the backbone therapy and the new treatment used in combination. But as the Office of Health Economics (OHE) has noted there is ongoing work and already a discussion on solutions.
The most promising solution seems to be adjusting the price for the constituents of a combination, termed multi-use pricing by the OHE. But even that solution is a challenge; OHE highlight the issue of whether payers or Health Technology Agencies (HTA) should or could be involved in price negotiations as well as how to do so whilst respecting competition law.
With NICE currently looking at 30 combination therapies, as identified on their website on 10 August 2021, the combination challenge will need to be tackled if patients are not to miss out.
About the author
Leela Barham is a researcher and writer who has worked with all stakeholders across the health care system, both in the UK and internationally, on the economics of the pharmaceutical industry. Leela worked as an advisor to the Department of Health and Social Care on the 2019 Voluntary Scheme for Branded Medicines Pricing and Access (VPAS).












