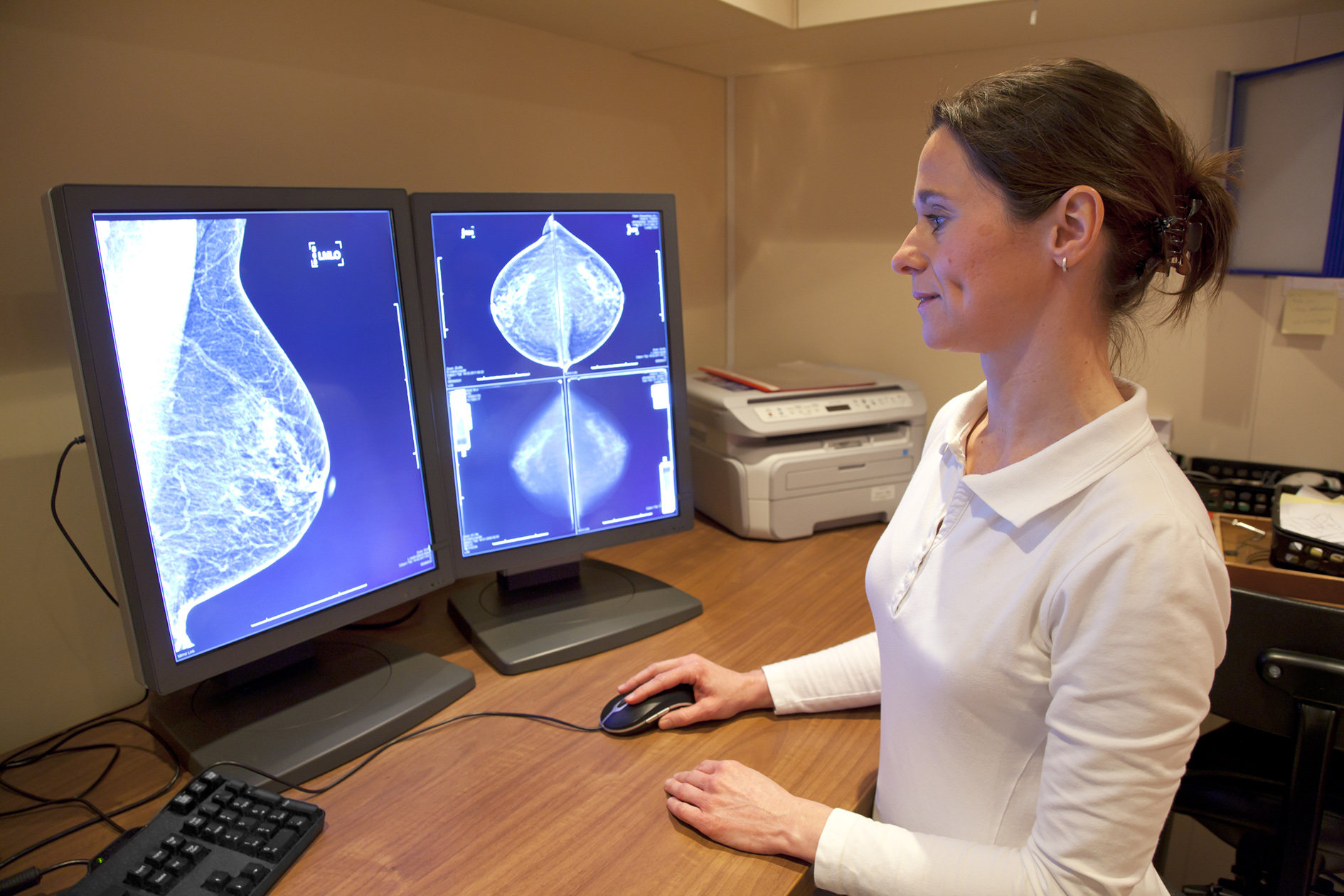
Survival data firms up Trodelvy prospects in new breast canc...
In the space of a couple of months, Gilead Sciences' Trodelvy impact on overall survival (OS) in the TROPICs-02 trial in advanced breast cancer has gone from a trend to a statistically sign