Why automated metadata management is important for simplifying the clinical trials process

The need for automated metadata management software has never been greater. The number of global clinical trials being run is increasing every year. And, they are becoming increasingly complex to manage. There’s a vast amount of data that needs to be collected and analyzed. There are more regulations in place than ever before and industry standards are constantly being updated.
Managing metadata effectively is challenging for organizations. Many are still using manual processes, such as Excel spreadsheets and Word documents, to manage standards. This is not efficient. It slows down the end-to-end clinical trials process. It’s error-prone. Difficult to manage. And it makes collaboration between stakeholders tough.
With the huge expense and complexity involved with running clinical trials, it’s more important than ever to stay ahead of the competition. Fortunately, there are technologies and best practices that help to simplify and streamline the end-to-end process.
View our free guide on how to increase trial efficiency with automated metadata management
What essential changes can be made to simplify the clinical trials process?
The most important changes that can be made are:
- To implement a metadata-driven clinical metadata repository (MDR)
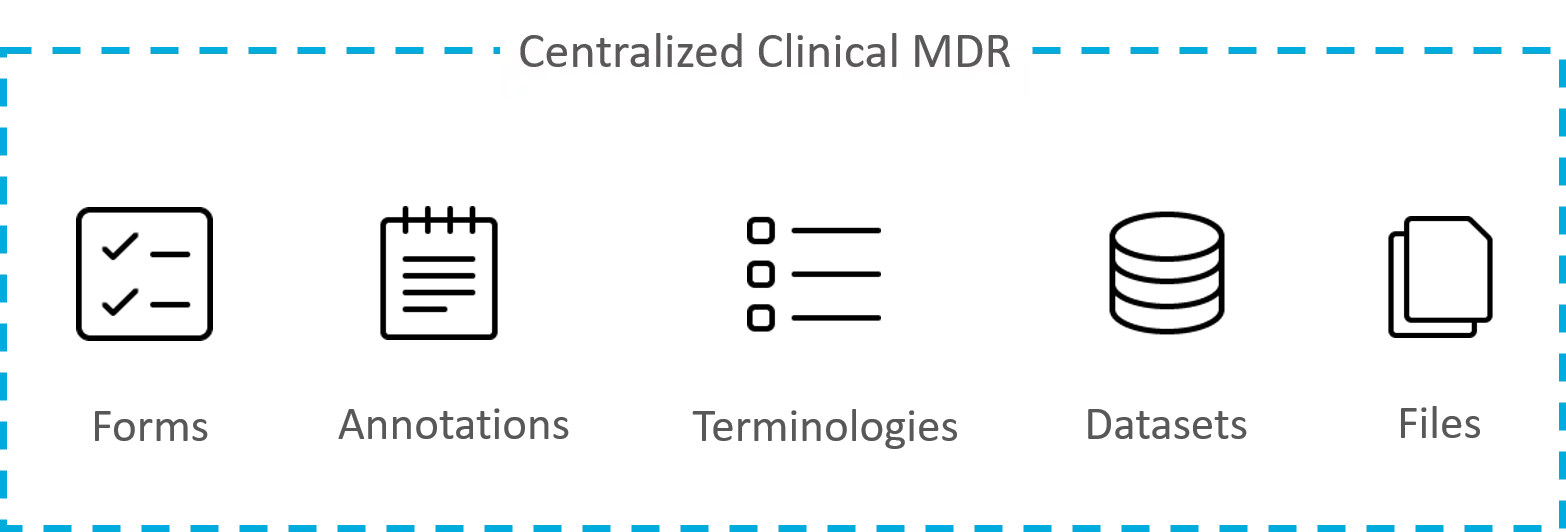
An MDR is a cloud-based centralized repository for storing and governing clinical metadata such as forms, datasets, and annotations. It has an interface that allows planning, communication, and collaborative working between different teams. Standards and automated processes are built into its framework to streamline clinical studies from inception to completion.
- To standardize clinical metadata
Standardizing metadata reduces the resources, effort, and time spent setting up studies. Once a metadata asset has passed quality checks, it can be reused across all existing and future studies. Metadata is more consistent, higher quality, and the likelihood of a successful submission is increased.
The benefits of implementing a clinical MDR with metadata-driven processes
Here are some of the key features and benefits to expect in a clinical MDR.
End-to-end metadata management
Cloud MDR systems facilitate end-to-end metadata management. Standardized metadata is stored in 1 place, so stakeholders can find what they’re looking for quickly and easily. There’s no need to trawl through different systems looking for the correct version of an asset.
Creating metadata from scratch takes a lot of time and is error-prone. The ability to reuse standardized metadata reduces the chance of errors, achieves consistency, and saves time and effort.
Studies transition through various stages, such as form design, EDC build, and dataset creation. Each stage in a clinical MDR is built with the next stage in mind. Standards such as SDTM, ADaM, and Define are integrated into the framework. This allows for seamless automation and inheritance between the different stages. For example, generating database specifications from form designs or SDTM conversion.
Using a cloud MDR improves management, planning, and communication. It’s also easier for clinical teams to establish and implement organizational governance processes.
Collaboration
Clinical MDRs allow full collaboration between teams in different locations. They’re built with stakeholders in mind to allow them to do their job effectively. Better communication between data managers, biostatisticians, standards managers, clinical operations teams, and external users, improves business processes and transparency.
Impact Analysis
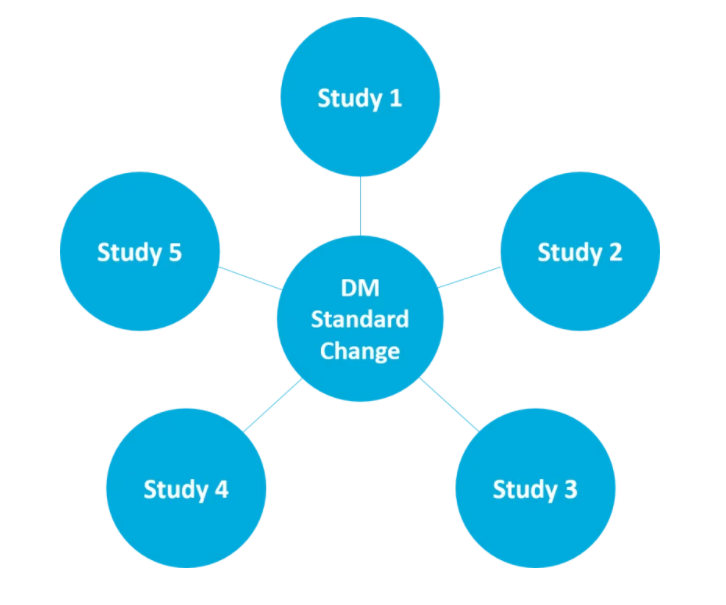
Impact analysis improves planning, decision making capability, and helps to ensure consistency between standards. It enables users to see the impact of upstream changes on downstream standards and makes it easy to keep track of where metadata is used. For instance, seeing what the impact of a change on a standardized form will make on SDTM datasets. Or, seeing which studies a specific version of a form is used in. Knowing the full scope of the impact of a change allows better decisions to be made about whether it’s worth implementing that change or not.
Change Control and Governance
Users can request changes to metadata such as adding, editing and retiring metadata assets. All changes are tracked and managed by workflows that define the approvals process - including who requested the change, what the change was, why it was made, and when. It’s easy to see the differences between metadata assets. This helps with consistency issues and having full end-to-end traceability ensures audit compliance.
Versioning
A clinical MDR should be able to maintain different versions of the same standard that has been updated or customized. This allows different versions of standards to be used in different studies. Greater visibility lets users see which version of a standard has been used in a particular study, and ensures that the correct version of a standard is being worked on.
Validation
Every clinical MDR should have validation built in every step of the way. Validation and reporting highlight any compliance deviations from regulatory standards, and alert users as to where issues reside. This makes it much easier to comply with standards and gives confidence that submissions are correct and complete.
Summary
By standardizing metadata and implementing a cloud MDR, the clinical trials process is much simpler and easier to manage. Governance, automated processes, built in standards, and validation increases process efficiency. Standardizing metadata assets allows them to be reused over and over again. The result? Saved time and costs, and better data handling capability. The quality of metadata and clinical trial data will be higher. And, that means faster, more successful study submissions!
How Formedix can help
Formedix has been helping organizations with clinical trials for over 20 years. Our clinical MDR platform has been built through a wealth of industry knowledge and a deep understanding of customer needs. It incorporates all the features outlined above, and more, to get you to submission faster and more efficiently than before. It’s fully integrated with all the different versions of CDISC standards and is aligned with all their updates. Quality checks enforce CRF, EDC, and CDISC compliance from beginning to end.
It’s off-the-shelf, so you can get started immediately. And, if you need it customized to meet your specific needs, we can do that too.
Our Professional Services team are experts in CDISC standards and are here to help you use our platform every step of the way. You’ll get an account manager dedicated to you for product support right from the start. And, if you have any tasks you want turning round quickly, such as CRF designs, EDC study builds, SDTM conversions, and define.xml for example, we can do that too. If you want to find out more about how we can help simplify and streamline your clinical trials process, give us a shout.