ODM standard for CRF design

Over the last few years, our industry has become familiar with CDISC standards. This has largely been driven by regulation, with national regulators such as FDA and PMDA mandating the use of submission standards such as SEND, SDTM, and ADaM.
This post shines a spotlight on the lesser-known Operational Data Model (ODM) standard, which is often overlooked as it’s not required by any regulators. Why should you be interested in it? Because it can be used as a standard way to define forms, independent of the data collection system. These forms can then be turned into organizational standards, driving data quality and consistency. Finally, they can be used to drive your data collection system. In short, they put you in control.
With the right tools in place sponsors and CROs can define data collection standards while remaining flexible in their EDC choice. They can also take control of their EDC build to save time and money. Standardizing your data collection means greatly reduced future study build cost due to reuse and increased consistency between studies.
If you are interested you can read our blog on Everything you need to know about CRFs.
ODM-based forms
As mentioned in the introduction to CDISC standards, one of ODM’s primary uses is to describe the data collected as part of a study. For the purpose of this article, we’ll focus on CRFs.
CRFs consist of groupings of questions, controlled terminologies, and some dynamic behavior such as edit checks, conditional logic, and derivations. ODM provides a standardized way of describing these objects in XML so they can be understood by any system that reads ODM. Thankfully there are form design tools available, so you don’t need to understand XML. You just create the form and the tool does the rest.
The core ODM model standardizes the 80% sweet spot of form design (e.g. question text, datatype, controlled terms), with the remaining details being left to the capabilities of each data collection system. For example, system-specific layout capabilities and complex edit checks. The ODM standard can be extended to support these system-specific capabilities, and Formedix has developed such extensions to fully support study build in many of the leading EDC systems.
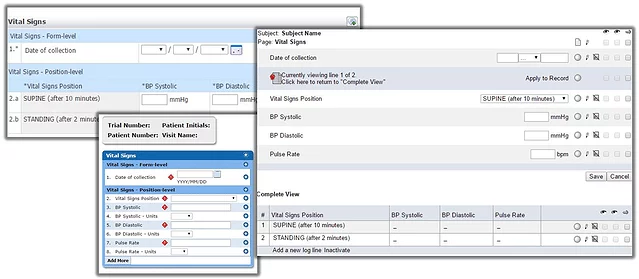
With advanced EDC integrations, you can design CRFs that use the full capabilities of your chosen EDC system, and view what the CRFs look like instantly at design time. What you review are the actual forms, so there’s no manual interpretation to introduce mistakes or elongate your study build. What you see is what you get.
Here's an example of a form viewed as it would look in Medidata Rave, Oracle InForm and Parexel DataLabs, all generated from a single ODM-based form specification:
Using ODM with CDASH
ODM is a data model rather than a content model, meaning it says how you describe forms but doesn’t define any actual standard form content. This is where the Clinical Data Acquisition Standards Harmonization standard (CDASH) comes in. The mission of CDASH is to harmonize your data acquisition with your SDTM submission. The “2.0” version is a major improvement, providing a core model that standardizes how to design forms that are aligned to the SDTM core model and an implementation guide that defines forms that match the domains in the SDTM implementation guide.
Using CDASH to design your CRFs not only gives you guidelines for what data to collect but ensures that it’s collected in a manner that’s consistent with SDTM. This means there’s a reduced risk of collecting poor or inadequate data. It also makes it simple to convert the collected data into SDTM, greatly reducing the cost of data mappings and conversions.
ODM and CDASH together give you the ability to define standardized forms that can be used across EDC systems and map very easily to SDTM. We can help you make use of CDASH to design SDTM-friendly forms that are optimized for your EDC system.
Building EDC systems
After creating your CRF design the EDC database then needs to be built. This is can sometimes be done by sponsors, but more often is handed over to a CRO or an EDC provider. The problem here is that the EDC build is traditionally a manual process. This means someone interpreting the CRF specifications and coding the eCRFs. This is time-consuming and error-prone, often taking many weeks over multiple frustrating review cycles.
It doesn’t have to be like this! If your study design is in an ODM-based design tool it becomes possible to integrate with ODM-friendly EDC systems to remove some of the manual processes.
Furthermore, if you’re using a study design environment with advanced EDC integration it is possible to automatically build the EDC system. This not only gets you an instant EDC build, but ensures it is exactly as you have designed with no mistakes. Misunderstandings become a thing of the past.
Benefits of standardization and reuse
Simply reusing your CRFs can save 68% in study setup time, because:
- There is no need to design CRFs again for each study.
- There is less QA overhead.
This also leads to downstream savings in SDTM conversion as data is more consistent between studies.
The next step is to define end-to-end organizational standards that span across studies and therapeutic areas to bring an extra level of consistency and savings. Think of this as strategic planning. Focusing on optimizing your whole set of clinical programs rather than thinking study-by-study. Rather than just standardizing forms, also standardize the datasets, mappings, and other metadata. When you have this, building your studies becomes vastly quicker as selecting the required forms can bring in all the related content such as downstream datasets and mappings. With this comes increased data quality and reduced validation effort.
Using ODM for CRF design rather than proprietary metadata formats makes this standardization and reuse highly effective, as the standards can be reused across systems and organizations.
Find out more about CRF design and standardization.
Therapeutic Area Standards
When designing new forms, perhaps for a new therapeutic area, it can be difficult to understand the considerations you should have when defining the forms and submission datasets. How should you optimally collect a particular type of data? Which pieces of data are important to collect to ensure your submission has everything it needs?
Thankfully CDISC has teamed up with the Critical Path Institute to form the Coalition For Accelerating Standards and Therapies (CFAST), a group dedicated to standardizing how CDISC standards should be used across a number of therapeutic areas. To date, they have published Therapeutic Area User Guides (TAUGs) for around 30 different areas such as Alzheimer’s, Influenza, and Prostate Cancer. These are invaluable resources when designing your studies.
Want to know more?
Formedix has been using CDISC to help drive efficiencies in clinical research for nearly twenty years, and have made major contributions to the development of the standards to ensure they provide maximum benefit to the industry.
Our clinical trial automation software and clinical metadata repository allows you to take advantage of CDISC standards and optimize your trial process.
If you have any questions about CDISC or would like to know how you can use it to your benefit, we have a team of experts that can help you use CDISC to accelerate your clinical trials. We can also provide various types of CDISC training to help get you up to speed.












