Oxford Biomedica signs ophthalmology R&D deal with Santen
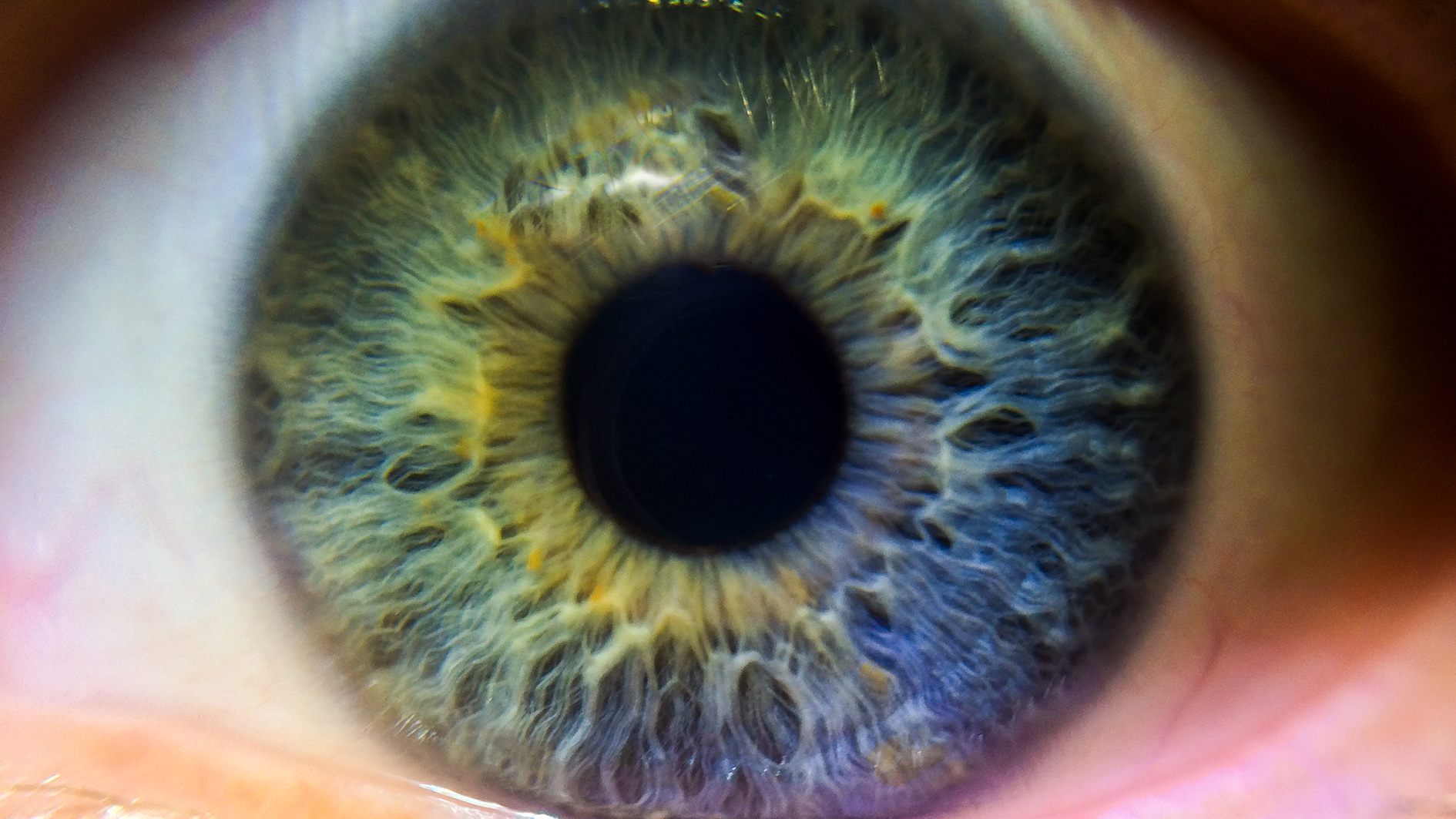
UK biotech Oxford Biomedica and Japan’s Santen have begun an option and licence agreement to research and develop gene therapy products for an inherited retinal disease.
Inherited retinal diseases are a group of rare disorders caused by one of more than 260 different genes where mutation results in vision loss or blindness, often disproportionally affecting children and young adults.
Spark Therapeutics has been a trailblazer in this field, becoming the first company to get a gene therapy for a rare inherited eye disease approved by the FDA in late 2017.
A single dose of Luxturna can improve the vision of people with confirmed biallelic RPE-mediated inherited retinal disease, although it cannot produce 20/20 vision in the condition that causes everything to seem shadowy and indistinct, particularly in low light conditions.
There are a host of other small biotechs developing gene therapies for rare eye diseases, including Nightstar Therapeutics, a UK firm that Biogen is acquiring for $877 million.
Oxford Biomedica has already made its name by providing the viral vectors used in Novartis’ CAR-T cancer cell therapy Kymriah, and is now looking to expand into other areas.
The company said inherited retinal diseases are ideal candidates for gene therapy because many of the responsible genetic mutations have already been identified.
As Spark has already proved, the eye is a readily accessible organ conducive to direct delivery of gene therapy vectors to the diseased tissue.
The aim of the R&D collaboration with Santen is to generate pre-clinical proof of concept to treat an inherited retinal disease with lentiviral vectors developed and manufactured by Oxford Biomedica (OXB).
The collaboration includes a licence to use OXB’s LentiVector technology and access to its industrial-scale manufacturing capabilities.
Oxford Biomedica is entitled to an undisclosed milestone payment on exercise of the option to the LentiVector platform as well as development milestones and up to a 10% royalty on net sales.
Santen has worldwide commercial rights to the programme, while OXB retains an option to co-fund and participate in development and commercialisation in the US and Europe.












