New guidance covers AI-based biomarkers for cancer care
Schematic for ESMO Basic Requirements for AI-Based Biomarkers In Oncology (EBAI).
AI technologies are promising to transform the care of people living with cancer, but like any new technology, there is a need for standardised ways to assess them to make sure they are fit for purpose.
Towards that end, the European Society for Medical Oncology (ESMO) has developed a new guidance document that outlines the minimum requirements for the validation and clinical use of AI-based biomarkers used for cancer diagnosis, prognosis, and treatment selection.
The ESMO Basic Requirements for AI-based Biomarkers In Oncology (EBAI) guidance – just published in the Annals of Oncology – provides "the first comprehensive guidance for the safe, trustworthy and effective integration of AI-derived biomarkers into cancer care," according to the organisation.
At last month' ESMO annual congress, the society also released guidance on the use of large-language models (LLMs) in clinical practice – dubbed ELCAP – to help patients, clinicians, and institutions use tools like ChatGPT responsibly for tasks like education and patient support, healthcare provider decision support and documentation, and extracting data from electronic health records.
"AI systems can function as biomarkers because they are able to analyse complex, multidimensional data to predict disease features and clinical outcomes, including treatment responses in patients with cancer," explained Jakob Kather, deputy chair of the ESMO Real World Data & Digital Health Task Force, who is one of the authors of the guidance.
"As AI technologies increasingly permeate oncology – from pathology and imaging to genomics and electronic health records – there is a growing urgency to ensure that AI-based biomarkers meet robust validation criteria before being used to inform treatment decisions," he continued.
"While there are hundreds of papers and dozens of products using AI to measure biomarkers, there has been no conceptual framework, no guidance on how to validate and use them. That's precisely what EBAI aims to provide."
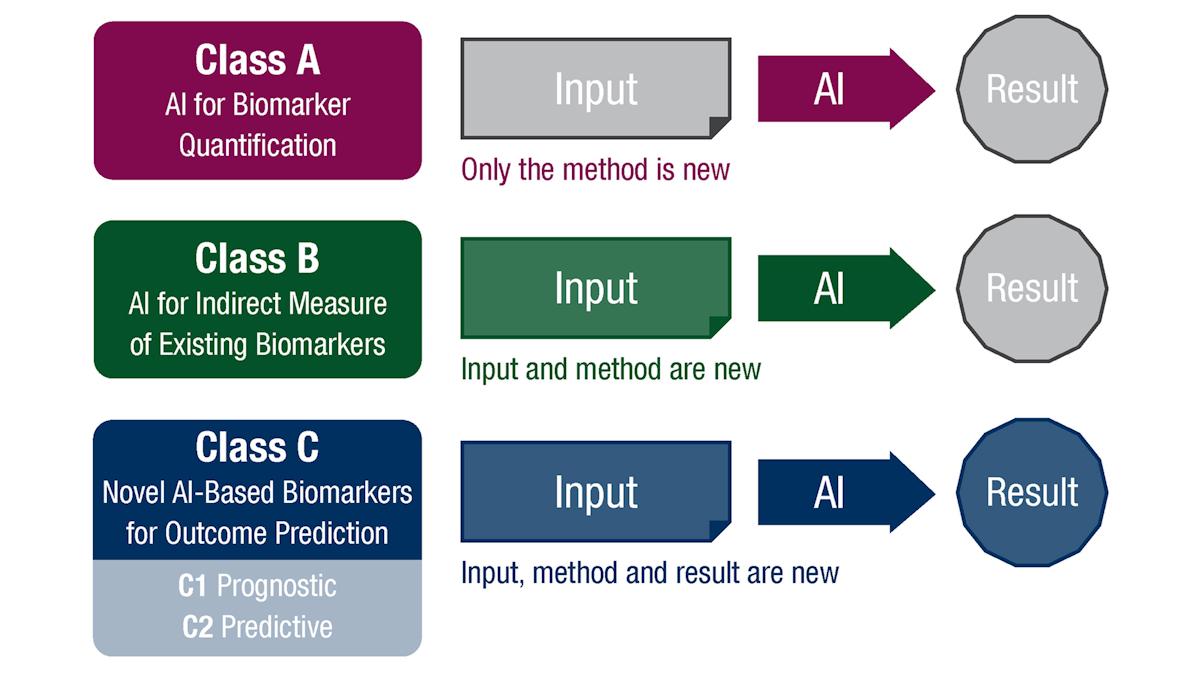
EBAI introduces three biomarker classes, each with tailored validation requirements, ranging from concordance studies to retrospective and prospective clinical trials.
Class A quantifies existing biomarkers using the same input data as standard biomarkers/assays, while Class B covers AI systems that act as indirect measures of known biomarkers, often used for screening.
"Imagine screening hundreds of thousands of patients using AI on histology slides, then confirming only the positives with molecular tests," commented lead author Mihaela Aldea of the Institute Gustave Roussy in Villejuif, France.
"That's a scalable, cost-effective approach, that could be applied and have a global impact," she added.
Finally, Class C encompasses novel AI-derived biomarkers that are trained directly on clinical outcomes, split into C1 (prognostic) and C2 (predictive) categories.
The hope is that EBAI will become a starting point for regulatory discussions, clinical implementation strategies, and industry development efforts.
"It's about finding the right balance – avoiding mistrust that delays progress but also over-reliance without reflection," said Aldea. "For that, respecting quality standards is essential."












