Innovent gets OK for China's first home-grown IL-23p19 drug
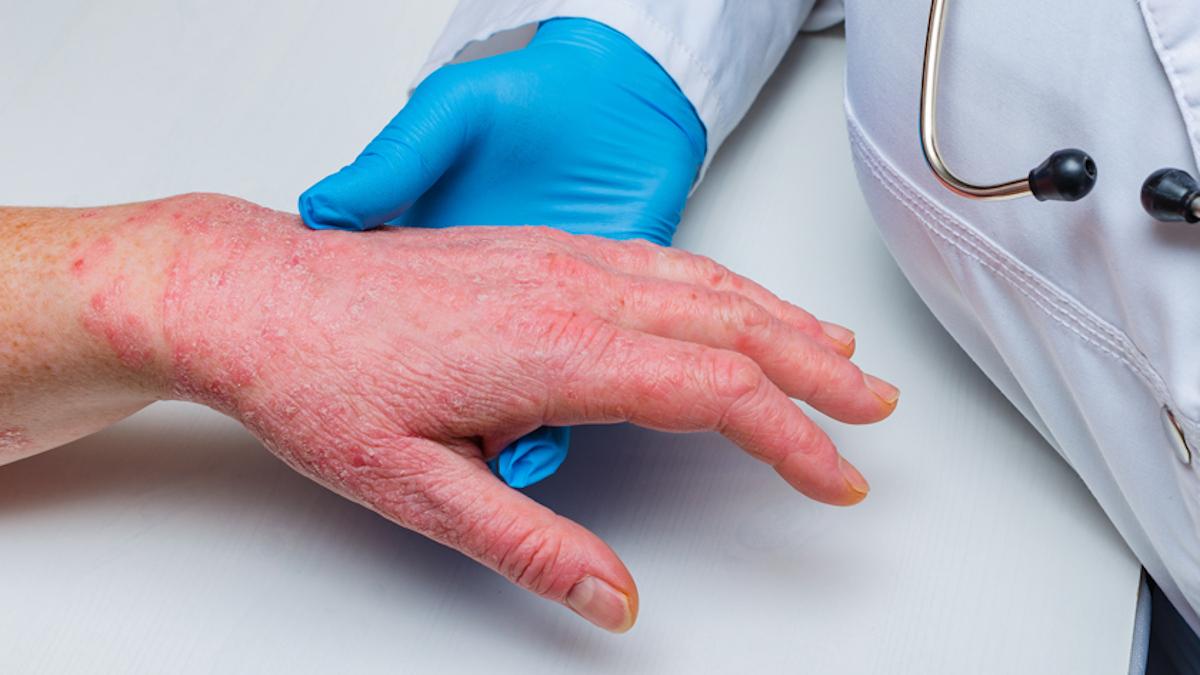
Innovent Biologics has picked up approval in China for Pecondle, its anti-IL-23p19 antibody, as a treatment for moderate-to-severe plaque psoriasis in adults.
Pecondle (picankibart) is the first drug in the class developed by a Chinese company to be approved by the National Medical Products Administration (NMPA), said Innovent.
It also offers dosing in the maintenance treatment phase of just one subcutaneous injection every 12 weeks, which is the longest treatment interval in the class among biologics used for psoriasis in China, according to the Suzhou-based company.
The drug is something of a 'pipeline-in-a-programme' for Innovent, which sees it as the cornerstone of an immunology and inflammation franchise, with trials already ongoing in ulcerative colitis, including a positive phase 2 readout, and studies in other indications planned.
Its approval by the NMPA comes on the back of the CLEAR-1 study, run in around 500 Chinese subjects, who received a 200 mg once-monthly dose of the antibody for the first 12 weeks, followed by a maintenance dose of either 200 mg or 100 mg every 12 weeks.
The primary endpoints were the percentage of patients achieving at least a 90% improvement in their Psoriasis Area and Severity Index (PASI 90) and of those achieving a static Physician's Global Assessment (sPGA) score of clear (0) or almost clear (1) at 16 weeks.
All told, 80% of Pecondle-treated patients achieved a PASI 90 result, compared to 2% of the placebo group, while for sPGA 0/1 the result came in at 93.5% and 13.1%, respectively. The benefits were also durable, with 84.9% of patients on the 200 mg maintenance dose maintaining PASI 90 at 52 weeks, and 85.9% of them still recording a sPGA /0/1 score.
According to Innovent, there are around 7 million people living with psoriasis in China. In recent years, the emergence of effective biologic medicines for those with moderate to severe symptoms of the skin disorder has meant that the previous target of achieving a PASI 75 response has been replaced with the more ambitious PASI 90.
"With its balance of efficacy and safety and convenient long-interval maintenance dosing, picankibart demonstrates strong potential as a best-in-class treatment," said Innovent's chief R&D officer for general biomedicine, Dr Lei Qian.
In July, another Chinese company, Akeso, claimed NMPA approval for dual IL-12 and IL-23 inhibitor ebdarokimab as a treatment for moderate-to-severe plaque psoriasis in adults.












