Dupixent delivers in two skin disease trials
Sanofi and Regeneron have celebrated a pair of positive clinical trials in inflammatory skin disorders with blockbuster immunology therapy Dupixent that could both lead to new indications for the drug.
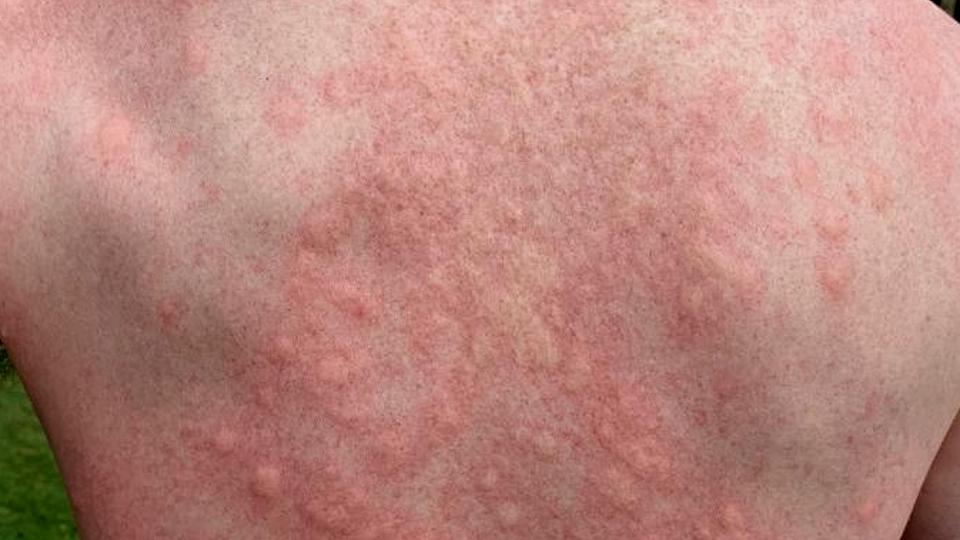
First up is the ADEPT study in bullous pemphigoid (BP), a rare skin condition that leads to reddening of the skin and the formation of painful and intensely itchy chronic lesions and blisters, in some cases spreading across a majority of the body. It most commonly affects older people in the 60 to 80 age bracket.
Dupixent (dupilumab) achieved a five-fold greater sustained remission rate at 36 weeks than placebo in the study – 20% versus 4% – meaning that the skin lesions had been resolved, with no relapse, and standard treatment with oral corticosteroids had been tailed off without the need for any rescue therapy.
The IL-4 and IL-13 inhibitor is the first biologic drug to show an improvement in remission and symptoms in BP, and the first to allow patients to reduce the use of steroids, which can have serious long-term side effects, said Sanofi and Regeneron.
In the 106-patient study, 41% of Dupixent-treated patients achieved a 90% or greater reduction in disease severity, compared to 10% of the control group, while "clinically meaningful" itch reduction was recorded in 40% and 11%, respectively.
"There is a significant unmet medical need for new medicines for people suffering with this hard-to-treat disease in which the standard of care is oral and topical corticosteroids and immunosuppressants – treatments that have poor clinical outcomes and safety concerns, respectively, and should be used sparingly in an elderly population," said Dietmar Berger, Sanofi's chief medical officer.
Sanofi and Regeneron said the data will support regulatory filings for Dupixent in BP around the world, starting with the US later this year.
CSU programme back on track?
The second trial – in chronic spontaneous urticaria (CSU) – is significant for the two companies, as it could resurrect Dupixent's chances of becoming a US-approved treatment for the condition after it was turned down by the FDA last October on the grounds that its efficacy had not been proven. The drug was, however, cleared for CSU – also known as chronic hives – in Japan.
The disorder affects around 1% of the global population and causes itching and swelling that can last for weeks at a time and resist even very high doses of antihistamines – the first-line therapy of choice.
The LIBERTY-CUPID C study involved 151 children and adults with CSU who added Dupixent onto antihistamine treatment. After 24 weeks, the Dupixent group saw an average 8.6-point reduction in itch severity on the 21-point ISS7 scale, compared to 6.1 points with antihistamines alone.
There was also a 15.86-point reduction in urticaria activity – itch and hives – with Dupixent versus 11.21 points for the control group, measured using the 42-point UAS7 scale.
The data will be shared with the FDA with the objective of refiling Dupixent for approval "as soon as possible", said the two drugmakers, which noted there are more than 300,000 people in the US with CSU that is inadequately controlled by antihistamines.
Treatment options for patients with the condition are looking up, with Novartis preparing to file for approval of its oral BTK inhibitor remibrutinib as a treatment for CSU, while Sanofi's rival oral BTK rilzabrutinib is in phase 3 for the condition.
Novartis/Genentech's anti-IgE antibody Xolair (omalizumab) has been a mainstay of treatment for refractory CSU for many years. However, the drug is heading for patent expiry in Europe this year and in the US in 2025.
There was some disappointment for Sanofi and Regeneron this week as well, however, after a small phase 3 trial in yet another skin disorder – chronic pruritus of unknown origin (CPUO) – failed to show a statistically significant benefit with Dupixent on the primary itch endpoint.
There were signals of efficacy in other itch-related measures, so testing is continuing in this indication.