ASH: Blenrep ups survival by 42% in multiple myeloma trial
GSK's BCMA-directed antibody-drug conjugate Blenrep reduced the risk of death by 42% in a multiple myeloma trial that the company believes could be practice-changing.
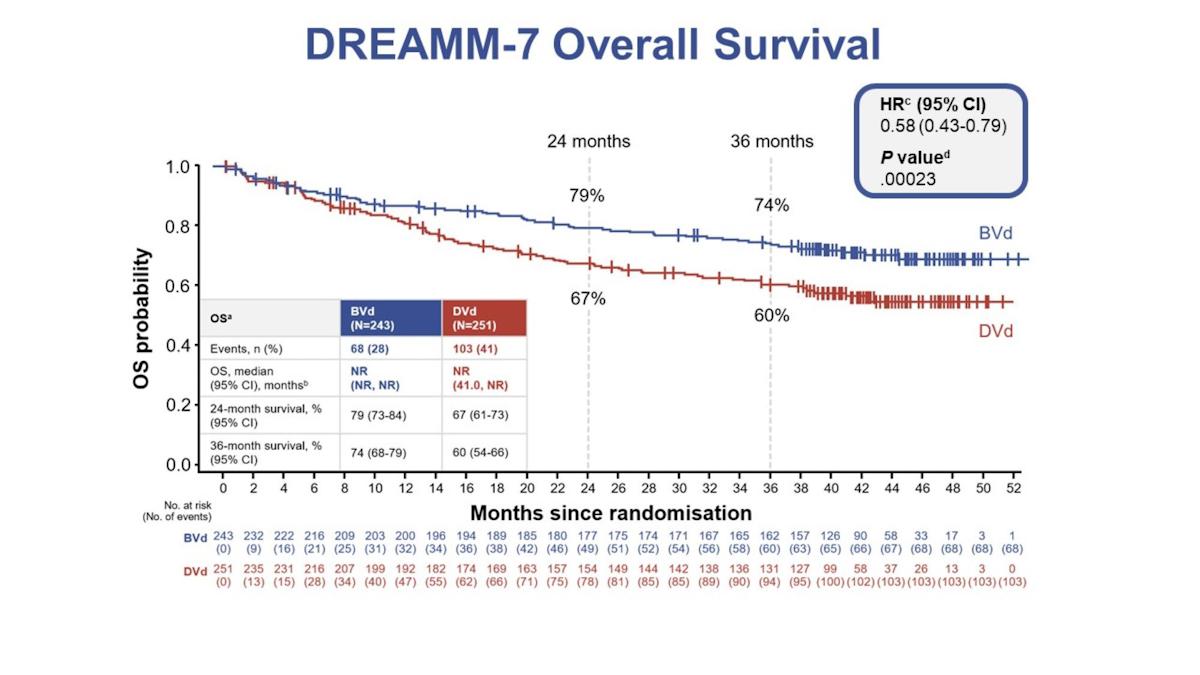
The DREAMM-7 study showed that Blenrep (belantamab mafodotin) was more effective than Genmab and Johnson & Johnson's anti-CD37 therapy Darzalex (daratumumab) – both given on top of Takeda's Velcade (bortezomib) plus dexamethasone (BorDex) – when used as second-line therapy for multiple myeloma.
The results were featured at the American Society of Haematology (ASH) annual meeting and, according to GSK, "support the potential for Blenrep combinations to become standard of care" in multiple myeloma patients at or after their first relapse.
The company previously teased the overall survival (OS) result and said it would be used to support regulatory filings for Blenrep, which was taken off the market in the US after the DREAMM-3 study failed to confirm its 2020 accelerated approval as a fourth-line or later treatment for the blood cancer.
After being all but written off, the DREAMM-7 results and accompanying data from the DREAMM-8 trial reported at this year's ASCO congress – which showed improved progression-free survival when Blenrep was added to Velcade plus pomalidomide and dexamethasone (PVd) after at least one prior line of therapy - suggest a renaissance could be coming for the product.
GSK's global head of oncology R&D, Hesham Abdullah, said the OS results represent "an important advancement that could redefine the treatment of relapsed or refractory multiple myeloma."
The data reported at ASH revealed a 74% OS rate in the Blenrep plus BorDex (BVd) arm of DREAMM-7 versus a 60% rate with Darzalex plus BorDex (DVd) regimen, with the difference between the two groups apparent within as little as four months after treatment started.
The median OS had not been reached in either arm of the study at the data cutoff point for ASH, but the investigators have predicted it would be 84 months for BVd compared to 51 months for the DVd group.
There was also a 2.5-fold increase over DVd on the proportion of patients with minimal residual disease (MRD) negativity – i.e., no detectable cancer cells – which was a key secondary endpoint of the trial. MRD negativity was achieved by 25% of the BVd group versus 10.4% of those taking DVd.
Principal investigator María-Victoria Mateos, of the University of Salamanca in Spain, told ASH delegates that the totality of evidence from DREAMM-7 represents a "potential paradigm shift" in the treatment of relapsed or refractory multiple myeloma.
GSK has also said it plans to continue working towards Blenrep's comeback with the pivotal DREAMM-10 study in newly diagnosed, transplant-ineligible multiple myeloma patients, which is due to start imminently.
The drug is still available in some ex-US markets and made modest sales of £3 million ($3.8 million) in the third quarter. That is well short of GSK's previous hope of turning the drug into a £3 billion blockbuster, which has now been restored, albeit not included in the company's financial forecasting.
While it was the first BCMA drug to reach the market, Blenrep is now facing competition in the category, particularly from BCMAxCD3 bispecifics like Johnson &Johnson's Tecvayli (teclistamab) and Pfizer's Elrexfio (elranatamab), which are approved as fifth-line or later multiple myeloma therapies, but which are in trials looking at earlier-line use.
Two BCMA-directed CAR-T therapies have been approved – Bristol-Myers Squibb's Abecma (idecabtagene vicleucel) and Johnson & Johnson's Carvykti (ciltacabtagene autoleucel) – also for later-line use.












