And then there were none: Regeneron cans last late-stage NGF drug
The hope that nerve growth factor (NGF) inhibitors could be an alternative to opioid for pain relief has finally been extinguished with the news that Regeneron had abandoned the last drug in the class.
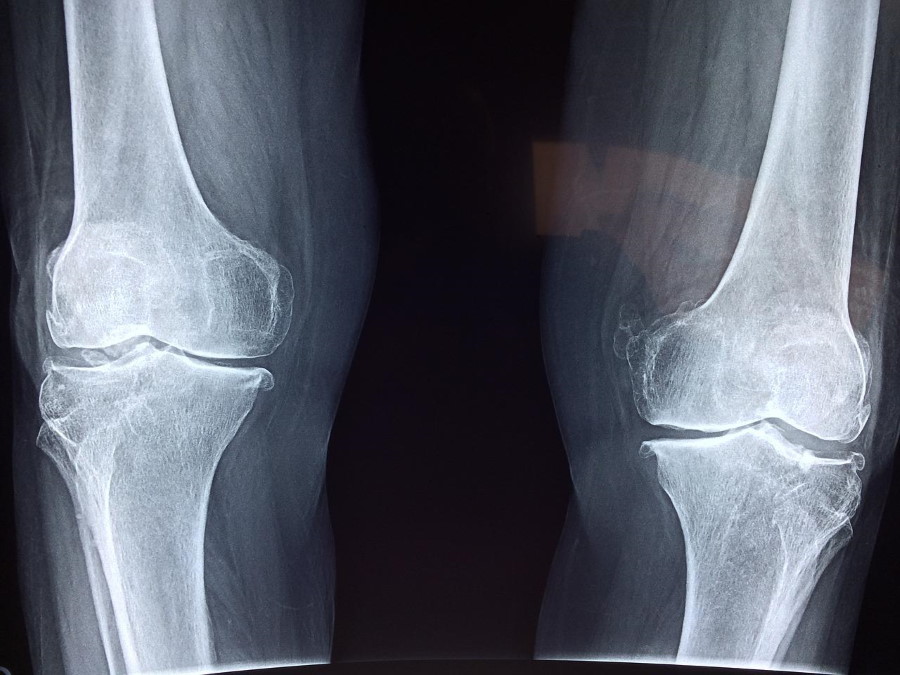
In its third-quarter results update, the company confirmed that it is halting further development of fasinumab, an NGF drug partnered with Teva and Mitsubishi Tanabe Pharma that was in phase 3 testing for osteoarthritis pain of the knee or hip.
It's a disappointing, but not unexpected, development for the programme, which was previously described by Regeneron as an "enormous opportunity balanced by a significant risk."
The number of NGF inhibitors in development has been steadily winnowed down in recent years, mainly due to toxicity issues, and until last year fasinumab was one of two remaining candidates in clinical testing.
Regeneron's main rival was Pfizer with tanezumab, which got as far as marketing applications in the US and EU for osteoarthritis-related pain, before being shot down by regulators on safety grounds.
The FDA rejected the drug last year after its expert advisors voted 15 to one against approval, and the EMA followed suit when its human medicines committee said its risks were too great, and there wasn't enough evidence to show it provided pain relief compared to standard non-steroidal anti-inflammatory drugs (NSAIDs).
Drugs in the class have been linked to cases of a rapidly progressing form of osteoarthritis, which can destroy joints entirely so they have to be replaced. Faced with the regulatory resistance, Pfizer gave up on tanezumab shortly afterwards.
Regeneron and Teva reported positive top-line interim phase 3 results with fasinumab in patients with chronic pain due to knee or hip osteoarthritis in 2018, but shortly afterwards had to halt the higher-dose arms due to toxicity issues.
The demise of fasinumab draws a line under the NGF inhibitor class, although there could be footnotes.
As of a September update, AstraZeneca was still developing MEDI7352, a bispecific antibody that combines NGF and TNF inhibition, potentially targeting both pain and inflammation associated with osteoarthritis. The drug started a phase 2 trial in 2018, but, while it was still listed as an active programme in the company's July pipeline update, there has been no news for some time.
Regeneron also said it was discontinuing a phase 3 study of REGN1908-1909, a multi-antibody therapy to Fel d 1 being studied as a treatment for cat allergy, due to futility.











