Alnylam launches digital tool for ATTR amyloidosis patients
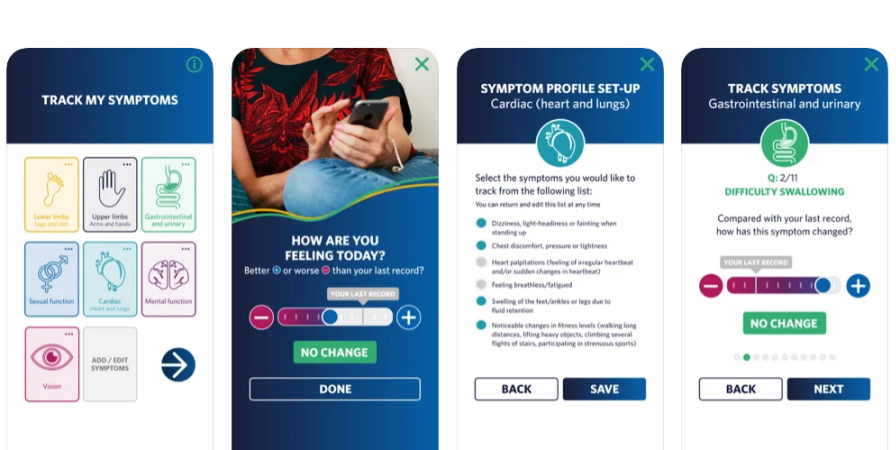
Alnylam has been a pioneer in hereditary ATTR amyloidosis, getting approval in 2018 for the first drug to treat the rare disease, and has now launched a digital companion to help patients track their symptoms.
The new app – called STAR – allows patients to record their symptoms and share them with healthcare professionals to encourage what the company says is "more confident and impactful discussions."
Hereditary ATTR amyloidosis is an aggressive, rapidly progressing, debilitating and fatal disease, and the launch of Alnylam's Onpattro (patisiran) was a milestone for patients and for the biopharma industry.
It was the first drug to be approved in the US that works by RNA interference – blocking the strand of RNA that transcribes a defective gene in a patient's DNA into a misfolded protein that leads to a disease.
hATTR amyloidosis is a complex disease because it affects multiple systems, including the heart, nerves, gastrointestinal tract and other organs.
Because the disease can evolve over time, "it is important for patients to accurately track any changes in their symptoms," according to Prof Philip Hawkins of the National Amyloidosis Centre in the UK.
"This new app will help people living with ATTR amyloidosis track their disease progression, while offering healthcare teams a more accurate picture of their patients’ disease," he said.
Alnylam says the free app is currently available for mobile phones on the App Store and Google Play in English as well as French, Spanish, Italian, German, Swedish, Danish, Portuguese and Dutch. A tablet format will be launched later this year.
"STAR was designed to help patients better understand their condition and its impact on their quality of life, including their ability to carry out day-to-day activities, mood, energy levels, and relationships with family and friends," said Dr Dinesh Kumar, Alnylam's medical director for UK and Ireland.
"Our goal is to empower patients to have greater control of their health and wellbeing," he added.
Around 1,350 patients with hATTR amyloidosis and nerve damage (polyneuropathy) worldwide were treated with Onpattro last year, and the drug generated $306 million in sales for Alnylam fuelled by its list price of around $450,000 per year in the US.
In Europe, Alnylam was beaten to the hATTR amyloidosis market by Ionis, which claimed approval for its antisense drug Tegsedi (inotersen) a few months earlier and also won an earlier endorsement from NICE in the UK for NHS use.
Alnylam meanwhile is running studies of Onpattro in hATTR amyloidosis characterised by cardiomyopathy, hoping to expand the label for the drug, and is also developing a second-generation therapy called vutrisiran which can be administered less frequently.
Vutrisiran is under regulatory review in Europe and the US, with decisions due in 2022.











