DIA 2024: Charting new horizons in patient-centered R&D

In its 60th year, the Drug Information Association (DIA) global annual meeting continues to be a critical gathering of the life sciences community.
From clinical trial sponsors to clinical research organisations, regulators, academics, and patients - all convene at DIA for transparent conversations about how to tangibly improve current care approaches. For those in clinical research and development, DIA gives us the opportunity to hear and share diverse perspectives on tackling the critical challenges of enhancing drug development for patients’ full journeys.
At this year’s meeting, there were several notable panel discussions and presentations regarding the digitalisation of clinical trials. Technology-enabled solutions to increase efficiencies and accelerate drug development for patients in need have been designed and are under development. However, there are multiple factors to consider for trial sponsors, sites, patients, and their loved ones, including how to minimise burdens of use, protect and ensure quality data, and ensure these technology-enabled solutions are purposeful and intuitive. We discuss some of these topics below.
The ongoing evaluation of clinical trial digitalisation
Following the increased use of tech-enabled solutions in clinical trials during and post-COVID-19, the industry has invested in examining the value of these capabilities in addressing ongoing challenges to drug development. Life sciences companies and technology providers aiming to meet patients where they are in their health journeys have dedicated resources to enhance the patient experience and quality of care in trials via a broad spectrum of technologies. These include:
- Patient-facing health and wellness applications, apps for self-management of disease, digital therapeutics, digital risk assessment tools, and digital biomarkers.
- Provider and patient interaction technologies, including care support applications, digital care programmes, remote patient monitoring apps, telemedicine, and remote electronic clinical outcome assessments.
- Provider-facing tools, such as comprehensive digital platforms, digital diagnostics, clinical diagnosis aids, and simplified document and compliance management tools based on digitisation.
However, as we add these patient-centered technologies to clinical trials, we must ask whether sponsors are seeing value from their investments, such as in terms of improving patient participation. As discussed at DIA, there is growing evidence that these digitised trial models are supporting sponsor goals in a host of ways critical to ensuring drug development is accelerated without compromising safety and quality for patients. Examples include:
- Decentralised trial models show costs reduced by 2% to 3% with four times the return on investment by using mobile technologies, telehealth, in-home visits, etc.
- 40% to 50% of pharmaceutical companies say artificial intelligence helps their drug development efforts. Specifically, for Phase I studies integrating AI-driven tools, there has been an 80% to 90% success rate compared to a 55% to 65% success rate for studies without AI.
- The use of digital health technologies to assess clinical trial digital endpoints is increasing quickly, and the integration of digital endpoints is providing ROI from 32% to 48% per indication for Phase II studies and four-to-seven times higher ROI for Phase III studies.
With an increasing number of digital capabilities supporting trials, it is vital that, early in trial design, trial sponsors and CROs gauge what technologies and digital approaches may benefit the trial, patients, and site teams without adding to their burdens or responsibilities. The true value of digital transformation is in learning to map patient and site journeys in ways that can enrich their experiences while advancing healthcare.
Imperative to reduce sites’ technology burden and enhance bandwidth
Global R&D spending on clinical technologies and services have increased in recent years as sponsors aimed to enhance operational efficiencies and prioritise patient-centred approaches. However, investigators and site teams say that, in some cases, the digitised tools and systems have added complexities to their day-to-day responsibilities. Sites with already overloaded staff are declining trials, which has slowed research.
Though many sponsors, CROs, and technology providers are providing custom strategies to help sites run their trials and use their products, investigators are using multiple technologies and processes to conduct trials for multiple sponsors and related vendors, which can be a lot to take on.
Technology consolidation under one roof
To ensure patients’ needs are addressed and integrated into trial design and execution, it is critical for sponsors, CROs, and service partners to collectively brainstorm on how best to support sites to ensure trials are started and completed, with quality care remaining the priority.
Just as industry stakeholders, including sponsors and CROs, are tearing down information silos and foregoing competition to improve diversity and inclusion in clinical trials and increase transparency regarding trial information and updates with the public, these stakeholders are recognising the site capacity challenge is bigger than one sponsor and its trials. For example, investigators and site teams are responsible for navigating a sponsor’s technology platforms, but also need to oversee patient visits, where they may need to set up electronic diaries or other technologies. Challenges with technology can cause patients to lose confidence or comfort with the trial process and drop out, which also falls on sites to help resolve. Recognising these challenges, how can sponsors and their partners help sites and their trials succeed?
As discussed by a DIA panel, what’s needed is a vendor-agnostic technology platform that simplifies access to site systems and tasks across trials. Currently, dozens of global pharmaceutical companies and clinical trial software vendors are being engaged to come together and provide sites with a single home for all the technology they need to get all their tasks done quickly, thus boosting their capacity and ensuring they can remain engaged in crucial research for the long term.
Through a single neutral platform, site teams may be able to:
- Use a single sign-on to access multiple sponsors’ software applications, whether to complete a feasibility survey to be selected for one sponsor’s trial, randomise a patient for a second sponsor’s trial, enter clinical trial data from a patient visit for a third sponsor’s trial, or set up participant stipends for a fourth sponsor’s study.
- See an automated, prioritised task list across sponsors, studies, and systems through a single dashboard.
- Quickly communicate with study teams when needed via chat.
By designing this type of intuitive platform with end-users in mind, sponsors and partners can also ensure site leads can spend less time onboarding new staff and trials because they can access everything they need through a single login.
As efforts to enhance site capacity and productivity continue, it will be worth monitoring the number of sponsors and tech vendors that join in, as critical mass will be necessary to meet the goal of reducing site burden and increasing site capacity to accelerate trials and bring new therapies to patients.
Understanding the patient experience via social media data insights
Trial sponsors are turning to a host of data sources (e.g., real-world data, tailored patient surveys, etc.) to dive deeper into understanding patients’ burdens, preferences, and experiences to better inform the focus of drug development investments and trial design.
And, as there are nearly 4.8 billion active social media users worldwide and growing, sponsors are also looking to these platforms to add to their data to further customise patient-focused data assessments, according to experts at DIA. As seen in Figure 1, on TikTok alone, there are more than 100 million cumulative user mentions of therapeutic specialties as of May 2023.
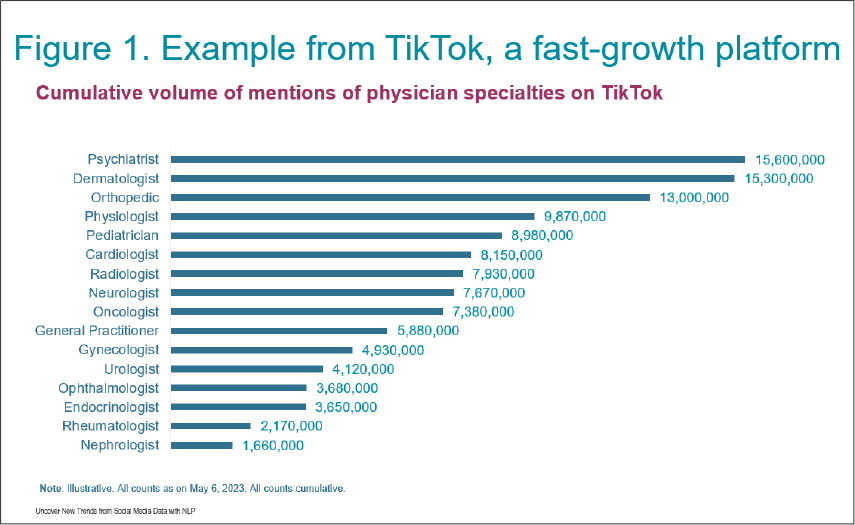
To extract valuable insights from this breadth of social media data, trial sponsors and CROs are relying on AI-driven techniques, including natural language processing, recursive training, large language models, and generative AI. Applying AI within an effective data governance and ethics framework is helping to fine tune social media listening activities while upholding individual privacy to gain drug development insights into:
- Treatment-related side effects and/or adverse events.
- Caregiver concerns for patients.
- Market gaps where patients need something that they are not finding solutions for.
When using social media data to fine tune patient-centred R&D approaches, sponsors should consider these challenges and pragmatic solutions for them:
- Large volumes of a wide variety of data: Social media conversational data is different from clinical data in its use of abbreviations, culturally distinct references and emojis, and its large volumes, varieties, and languages. Multiple AI-driven techniques can help to organise this amount of varying data. For example, in terms of topic analysis, clinical machine learning models can distinguish between patients and caregivers to contextualise information for accurate insights.
- Privacy and ethics: It is critical to consider social media users’ expectations for privacy within the applicable regulatory framework(s) and adhere to each social media site’s terms and conditions. Sponsors should also apply privacy protections, such as removing user identifiers and securing data throughout processing.
- Reliability of real-world data: The role of social media as a supplementary source of information for regulatory decision making, and even in clinical development, is yet to be clearly defined. This is partially due to how social media data introduces challenges in deriving cause-and-effect relationships to be considered as evidence. Within data strategies, using social media to generate hypotheses and to complement - instead of replace - other data sources will help to integrate these patient insights into trial design and balance important benefits with reasonable limitations.
To navigate challenges using social media data, practitioners can learn from successful case studies to further elevate the patient’s voice in R&D. Sponsors should establish a data pipeline for processing social media information that uses a multitude of AI/ML techniques within an effective governance framework to preserve privacy and promote a balanced approach to data reliability.
Continuing discussions and evaluations of trial digitalisation
Once again, coming together from around the globe to connect with fellow stakeholders and experts who share a common passion to positively impact patient lives at DIA was both inspiring and rewarding.
As we shift into a digital way of working in R&D and do so at a rapid pace, having more collaborative discussions where stakeholders look past individual goals will be critical to ensuring timely and quality drug development continues for those in need through development and integration of purposeful technology-based approaches.
About the authors
 Gareth Dabbs is VP of digital product & solution strategy at IQVIA. In his current role, he helps oversee the organisation’s technology portfolio and product strategies. With a background in R&D and commercial, he believes driving connection across the life sciences value chain is critical.
Gareth Dabbs is VP of digital product & solution strategy at IQVIA. In his current role, he helps oversee the organisation’s technology portfolio and product strategies. With a background in R&D and commercial, he believes driving connection across the life sciences value chain is critical.

Sarah Lyons is VP of data transformation and AI llatforms, real world solutions, at IQVIA . In her current role, she is responsible for serving healthcare and life science organisations globally with the platforms’ capabilities spanning privacy-enhancing technologies, natural language processing, and AI-enabled language solutions. She also participates in multiple industry forums and consortiums to promote data sharing across the healthcare ecosystem. Lyons was previously the head of Privacy Analytics, an IQVIA company specialising in privacy solutions for enabling the safe and responsible uses of data. She has been recognised as an HBA Rising Star by the Healthcare Businesswoman’s Association.
 Rajneesh Patil is VP of digital innovation at IQVIA. He also serves clinical operations at IQVIA as head of digital strategy and analytics innovation. Patil is a gold medalist in dental surgery from India with Foundations in Public Health from Australia and digital strategy-executive from Harvard Business School, USA.
Rajneesh Patil is VP of digital innovation at IQVIA. He also serves clinical operations at IQVIA as head of digital strategy and analytics innovation. Patil is a gold medalist in dental surgery from India with Foundations in Public Health from Australia and digital strategy-executive from Harvard Business School, USA.










