PhRMA 2013 Research & Hope Awards: Dr John Schiller

Rebecca Aris interviews Dr John T. Schiller of the National Cancer Institute, who was recently awarded, along with co-worker Dr Douglas Lowy, a 2013 Research and Hope Award for vaccines.
This week, the Pharmaceutical Research and Manufacturers of America (PhRMA) announced the recipients of the 2013 Research & Hope Awards, which honors outstanding achievements in vaccines research and immunization by individuals and research teams in the biopharmaceutical sector, academic / public research and health care provider communities.
"Over the last century, vaccines have transformed the public health landscape in the United States and around the world, preventing disease and improving the quality of life for multiple generations," said PhRMA President and CEO John J. Castellani. "PhRMA is proud to honor the recipients of this year's Research & Hope Awards. These inspiring collaborators within the biopharmaceutical ecosystem have helped drive the latest advances in vaccines and immunization to the benefit of patients everywhere."
pharmaphorum is pleased to interview here Dr John Schiller who, alongside Dr. Douglas R. Lowy, was awarded the PhRMA Research & Hope Award for Academic or Public Research in Vaccine Development for their work on developing the HPV vaccine.
Award recipient: Dr John T. Schiller and Dr Douglas R. Lowy
Company: National Cancer Institute
Award: The PhRMA Research & Hope Award for Academic or Public Research in Vaccine Development
Reason for winning the award: Dr. Douglas R. Lowy and Dr. John T. Schiller are receiving the 2013 Research & Hope Award for Academic or Public Research for the integral role they have played in the development of the human papillomavirus vaccine against cervical cancer, performing fundamental research regarding the nature of the virus, animal studies, and a Phase I trial in humans. Their research directly led to the first HPV vaccine, approved by the FDA in 2006, which has the potential to drastically reduce the incidence of cervical cancer in women.
Today, in addition to their ongoing study of papillomavirus – including the potential use of PV pseudovirus as gene transfer vehicles – Drs. Lowy and Schiller are working with colleagues at the World Health Organization and other organizations to find ways to distribute HPV vaccines to those in need.
Interview summary
RA: Can you please tell me about your work in the vaccines space that has led to you being awarded the 2013 Research & Hope Award for Academic or Public Research in Vaccine Development?
JS: Dr Lowy and I developed the L1 virus-like particle (VLP) technology underpinning the commercial HPV vaccines produced by Merck (Gardasil) and GlaxoSmithKline (Cervarix). These are the first approved vaccines that specifically target cancer and successfully target a local sexually transmitted infection.
RA: Can you talk us through the milestones that have led to the development of the HPV vaccine?
JS: We made two breakthroughs critical to the vaccine's development. First, the discovery that the L1 major structural protein of papillomaviruses could self-assemble into non-infectious VLPs, that had the ability to induce high-titer neutralizing antibodies and could be produced, using our methods, at the large scale necessary for a commercial vaccine. Second, for HPV16, the most important oncogenic type, we discovered that the main isolate in use worldwide at the time had a previously unrecognized L1 mutation preventing it from self-assembling. We identified wild type HPV16 L1 and used it to generate the VLPs crucial to the HPV vaccine.
We also developed the key serologic assays to evaluate the vaccine-elicited immune responses. We used these assays, and a mouse cervicovaginal challenge model for HPV we developed, to investigate the extraordinary efficacy of the current vaccines. The assays have also been critical in the evaluation of second-generation vaccines, that we and other groups have developed, that might have broader coverage and / or be less expensive to manufacture and deliver.
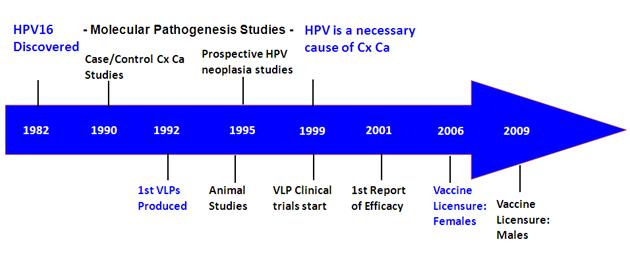
Figure 1: Timeline of HPV association with cancer vs vaccine development
In the figure of the timeline of important developments, it's important to note that there were two equally important tracts that progressed in parallel, the obvious one to develop and test the vaccine and the another one to identify the subset of HPV strains associated with cancers and to prove beyond a reasonable doubt that they are the central cause of cervical cancer.
"We made two breakthroughs critical to the vaccine's development."
RA: What impact could the HPV vaccine have on reducing the incidence of cervical cancer in women?
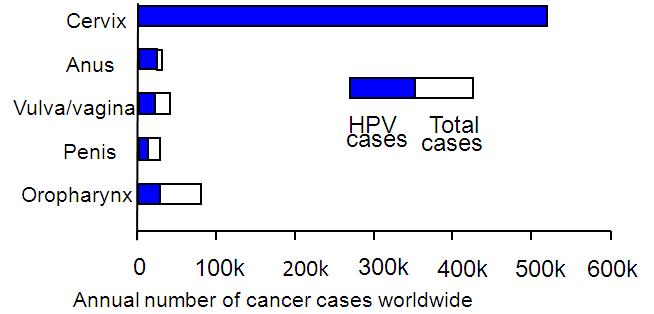
JS: Cervical cancer dominates the HPV-associated cancer burden worldwide, in large measure because most women in underdeveloped and emerging countries don't have access to screening. There are about half a million cases and a quarter of million deaths from cervical cancer worldwide. However, in countries with good cervical screening programs other HPV-associated cancers, e.g. anal and oropharyngeal make up a larger fraction of the burden. Since these are cancers that men also get, males contribute a larger minority of the HPV cancers in these countries.
Figure 2: Annual worldwide incidence and distribution of cancers attributable to HPV
HPVs cause 5% of all cancers – Source: de Martel, Lancet Oncol 13:607-15, 2012
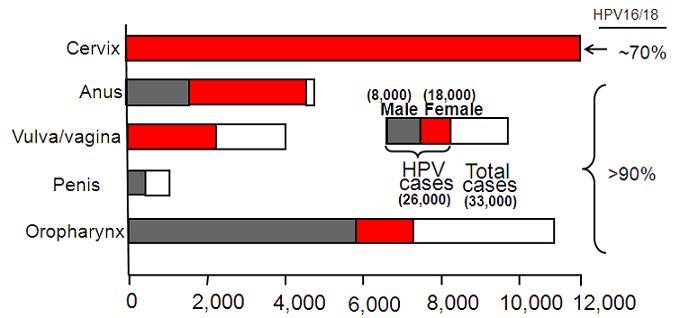
Figure 3: United States: Annual Incidence and Distribution of Cancers Attributable to HPV in 2004–2008
Pap screening has reduced the incidence of cervical cancer by ~ 80%
Incidence of HPV-positive oropharynx cancer 1988-2004 increased 225%
Source: MMWR Weekly 61:253-80, 2012
The two figures depict this difference. Two strains, HPV16 and HPV18 are found in 70% of cervix and 90% of other HPV-associated cancers. The vaccines target these two strains and protection is largely strain specific, although there is some partial protection against a few very closely related strains. So we would expect the vaccines to reduce a women's chance of getting cervical cancer by about 70-80%. Gardasil, the Merck vaccine also induces almost complete protection against the two strains, HPV6 and HPV11, that cause 90% of genital warts. Rates of genital warts are dropping dramatically in countries that have developed vaccination programs with high coverage rates, such as Australia and Denmark. This is true for young heterosexual men, in addition to young women, even though, until recently, they were overwhelmingly unvaccinated, presumably due to the herd immunity induced by vaccinating females.
RA: How can the HPV vaccine be made more widely available?
JS: In some developed countries, like the U.S., the issue is uptake of vaccines that are available. Many factors contribute to underutilization, including reluctance of some parents, lack of a strong recommendation by some pediatricians, anti-vaccine propaganda, and simply the inconvenience of having to go to a clinic to receive a series of three vaccinations. Clear and extensive public health messaging campaigns and well organized school-based delivery can overcome these barriers in many cases as demonstrated by the success of vaccination programs in the UK and Australia. In most of the world, the issue is availability, which needs to be attacked on several levels. Both Merck and GSK have agreed to tier pricing and sell the vaccines to GAVI for about $5.00 per dose. This is a major recent development. Vaccine manufacture in emerging countries might further increase supply and decrease cost. Lastly, the infrastructure for adolescent vaccination programs needs to be developed and validated before undertaking extensive public sector vaccination programs. Progress is being made in all of these areas.
"There is the obvious need to increase adolescent vaccine uptake worldwide"
RA: What would you like to see change in the HPV space over the next ten years?
JS: There is the obvious need to increase adolescent vaccine uptake worldwide, which would greatly reduce the incidence of cervical and other HPV-associated cancer in the next generation of women (and men). In addition to the approaches to increase vaccine availability noted above, I am also optimistic that the development of second generation vaccines or at least alternative vaccination protocols will increase the number of individuals that are effectively protected from HPV-associated cancer. But we also need to increase the effectiveness and coverage of cervical cancer screening and treatment programs for this generation of women. Inexpensive point of care screening tests based on direct detection of the virus DNA look most promising for the current generation of women who don't have access to quality cervical cytology (Pap) screening programs.
RA: What does winning this award mean to you and the team?
JS: Most importantly, the award provides an acknowledgment of the ability of our biomedical research enterprise to provide fundamental insights and practical solutions to important public health problems. I am confident in saying that the discovery of HPV infection as the central cause of cancer (for which Harald zur Hausen received the Nobel Prize) and the translation of this knowledge into an effective intervention were inevitable given the state of publically funded academic research and the technical capacities of the pharmaceutical industry at the time. It illustrates that although we might not be able to predict the timing and exact formulation of the next important public health intervention, we can feel confident that we moving forward in a broad based way toward achieving it.
About the interviewee:
Dr. Schiller graduated from the University of Wisconsin-Madison with a BS in molecular biology in 1975. In 1982, he received a PhD from the Department of Microbiology of the University of Washington in Seattle, and then joined the Laboratory of Cellular Oncology as a National Research Service Award postdoctoral fellow in 1983. Dr. Schiller became a senior staff fellow in the Laboratory of Cellular Oncology in 1986 and a senior investigator in 1992. In 1998, he became chief of the Neoplastic Disease Section of the lab. Along with colleague Douglas Lowy, he has been honored with the Albert B. Sabin Gold Medal Award and the Federal Employee of the Year Service to America Medal by the Partnership for Public Service
Dr. Schiller's research has focused on the basic biology and vaccine development for human papillomavirus.
The Pharmaceutical Research and Manufacturers of America (PhRMA) represents the country's leading innovative biopharmaceutical research and biotechnology companies, which are devoted to discovering and developing medicines that enable patients to live longer, healthier, and more productive lives. Since 2000, PhRMA member companies have invested approximately $550 billion in the search for new treatments and cures, including an estimated $48.5 billion in 2012 alone.
How can vaccine uptake be increased ?