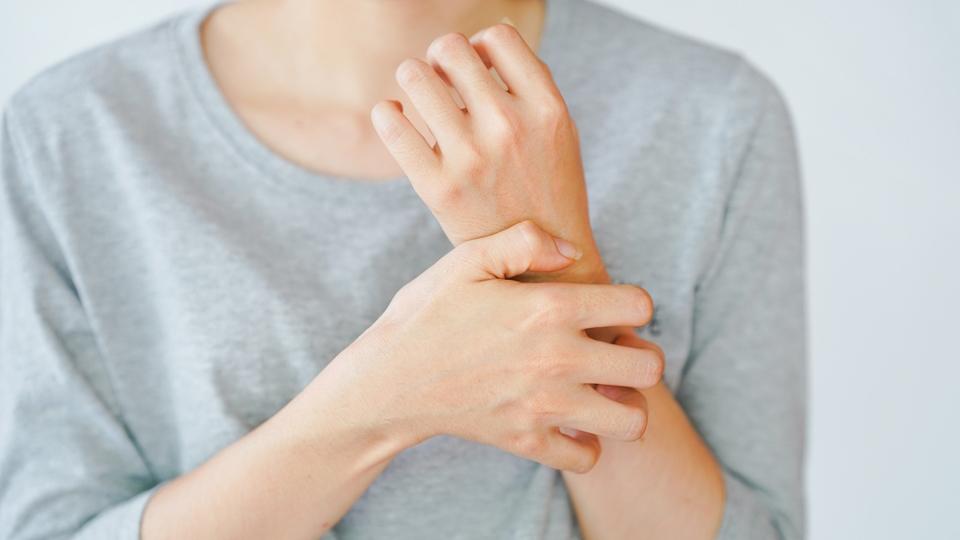
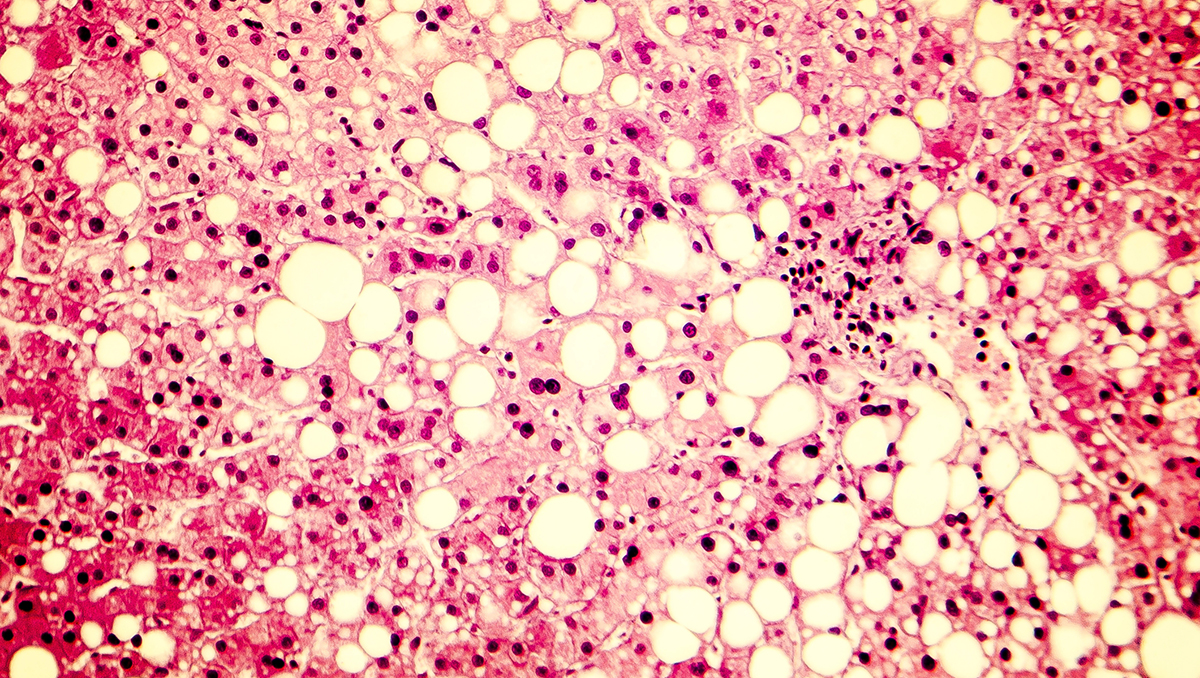
Pruritus in PBC: Understanding its impact and the need for r...
September is the perfect time to turn up the volume on conversations about Primary Biliary Cholangitis (PBC) and the symptoms people living with it experience, in support of PBC Awareness M