Another NASH setback as Akero’s efruxifermin stumbles
Around this time last year, Akero was riding high on positive phase 2b data for its candidate non-alcoholic steatohepatitis (NASH) therapy, but new data has dented optimism about the project.
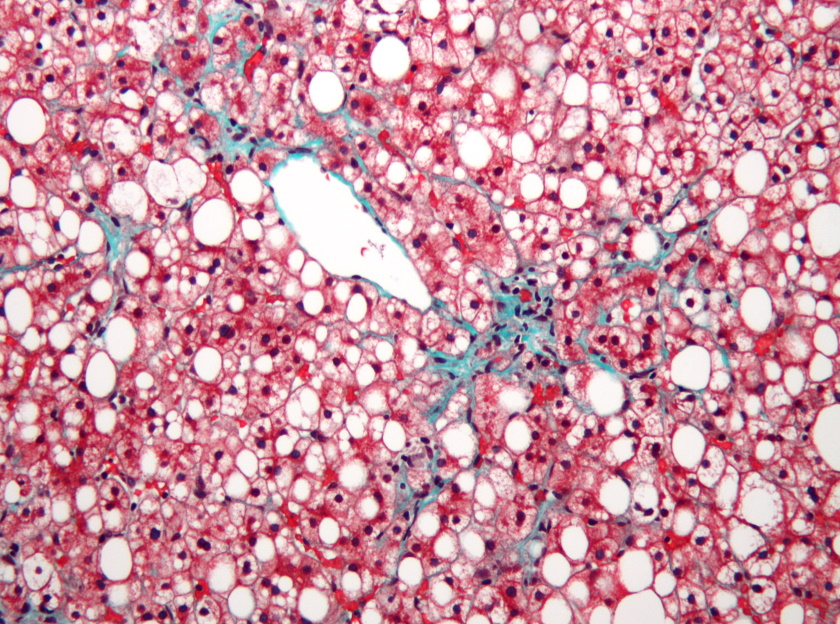
Results of another phase 2b study – called SYMMETRY – showed that efruxifermin was unable to hit its main objective as a treatment for patients with compensated cirrhosis (F4) due to NASH, a form of non-alcoholic fatty liver disease (NAFLD).
In the 182-patient study, patients were treated with one of two doses of the FGF21 mimic or placebo - given as a once-weekly subcutaneous injection - with the primary endpoint being the proportion of patients who achieved a one-stage or better improvement in fibrosis with no worsening of NASH.
Akero has put a positive spin on the results, saying that there were efficacy signals in the data that still point to a potential role for efruxifermin in NASH. However, investors were spooked by the announcement and shares in the biotech lost almost two-thirds (64%) of their value today.
The fallout affected rival biotech 89bio as well, that company also developing a drug in the FGF21 mimic class, and it lost more than a third of its value.
The biotech’s chief executive, Andrew Cheng, said the study had an ambitious primary endpoint, aiming to show an improvement in fibrosis after just 36 weeks. At that time point, 22% of patients given a 28 mg dose of the drug and 24% of those on a 50 mg dose reached that level of improvement, compared to 14% of the placebo group, with the differences failing to reach statistical significance.
Akero has highlighted some positive secondary data points, including that 4% of patients who received its drug at either dose had a two- or three-stage improvement in fibrosis with no worsening NASH, which was not seen in any patient in the placebo group.
In addition, 63% and 60% of patients in the 28 mg and 50 mg dose groups, respectively, saw resolution of NASH, compared to 26% of the placebo group, which Akero said was a significant difference and “the highest response rates reported to date for NASH resolution in this patient population.”
Last year, Akero trumpeted the results of the HARMONY study in less ill patients with stage 2 or 3 fibrosis, two thirds with diabetes, which met its primary and secondary objectives. In both studies, the drug was associated with mild to moderate and “transient” side effects, mainly gastrointestinal reactions like diarrhoea.
The company also noted that patients with cirrhosis due to NASH face a bleak prognosis, as, unless they receive a transplant, only half survive beyond five years.
There has been a string of failed projects and setbacks for companies developing drugs for NASH in the clinic, including candidates from Intercept Pharma, Gilead Sciences, Genfit, Albireo, CymaBay, Cirius, and NGM Biopharma.
“We believe this analysis of the SYMMETRY study contributes to a growing body of evidence for [efruxifermin’s] potential to benefit patients with NASH who are either cirrhotic or pre-cirrhotic,” said Cheng.
He added that looking at the data “in their totality” gives the company confidence that the drug will “show additional improvements for patients after the long-term follow-up period is complete at week 96.”
At the moment, Madrigal Pharmaceuticals seems to be in the lead to bring a NASH drug to market, after its daily oral drug resmetirom met primary and secondary objectives in a phase 3 NAFLD trial that included some patients with non-cirrhotic NASH.
A second phase 3 trial is due to report before year-end and, if positive, could lead to regulatory filings in the first half of 2023.