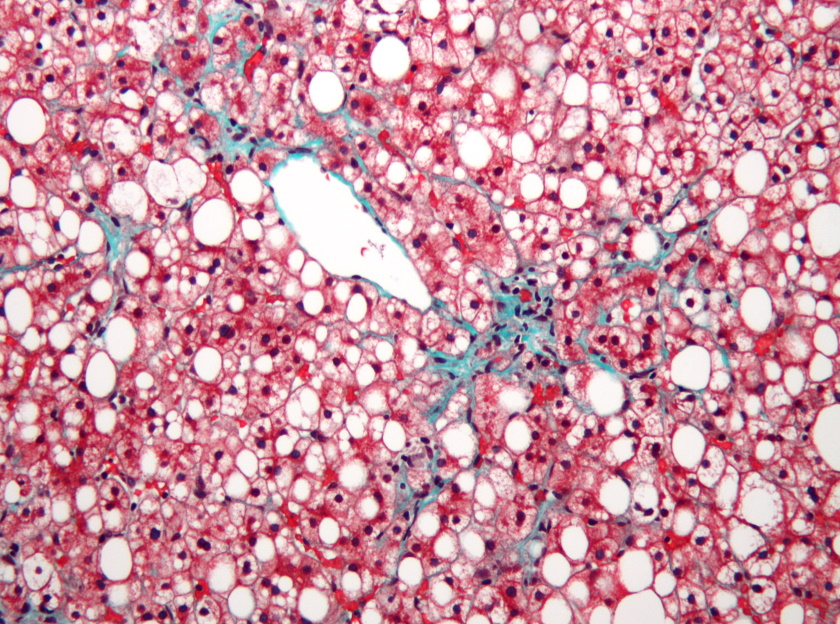
Intercept changes course as OCA is rejected for NASH again
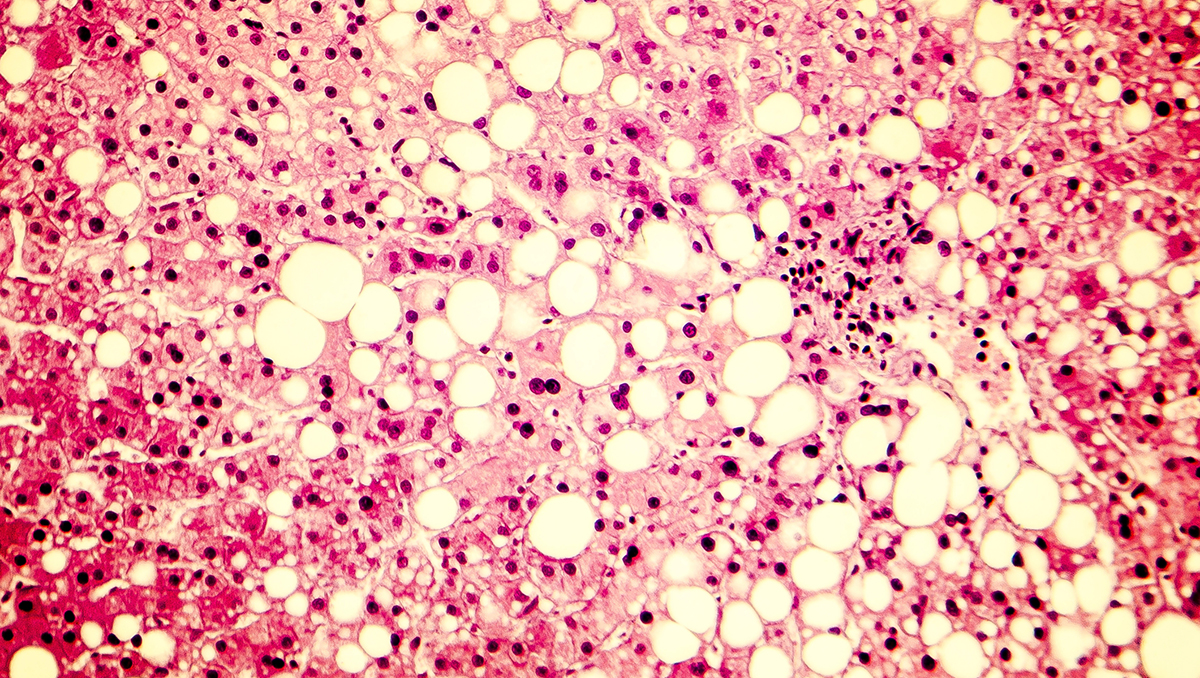
Intercept Pharma has conceded defeat in its battle to get obeticholic acid (OCA) approved as a treatment for non-alcoholic steatohepatitis (NASH) after the FDA rejected its marketing application for the second time.
Shares in the company fell sharply after the news emerged, ending Intercept’s hopes of bringing the first approved therapy for NASH to the US market and prompting it to abandon all activities in this disease area and refocus on “rare and serious liver diseases”.
The FDA – which previously rejected OCA in 2020 – said in a complete response letter (CRL) that the drug could not be approved as a treatment for pre-cirrhotic fibrosis in NASH patients based on the current data.
The drug would need to be refiled with long-term outcome data from Intercept’s phase 3 REGENERATE study “as a minimum”, said Intercept. Faced with that uncertainty, the company has decided to throw in the towel.
Chances of approval were already vanishingly slim after an FDA advisory committee voted against approval of the OCA last month, saying its benefits did not outweigh its risks and asking for new data from REGENERATE, which will now be discontinued. Safety issues identified with the drug included liver injury, a possible increased risk of diabetes, and tolerability issues such as itching.
Intercept already has a revenue stream in place from sales of OCA in a lower-dose formulation, branded as Ocaliva, that is used to treat primary biliary cholangitis (PBC) and made sales of $68 million in the first quarter of this year. And, on the plus side, Intercept said that shedding the costly NASH effort will allow it to reach profitability in 2024.
Today, the company reported new data showing the benefit of combining Ocaliva with pan-PPAR agonist bezafibrate in a fixed-dose combination in PBC. Bezafibrate isn’t approved in the US, but has been used for decades elsewhere as a cholesterol-lowering agent, and the new combination product could help Intercept expand its PBC franchise.
Ocaliva, meanwhile, is also in human testing as a treatment for biliary atresia, another rare liver disease. At the moment, Intercept’s only other clinical-stage candidate is INT-787, a farnesoid X receptor agonist in a phase 2 study involving patients with severe alcohol-associated hepatitis (sAH).
Next up in the roster of companies trying to bring a NASH drug to market is Madrigal Pharma, with its resmetirom candidate, due to be filed for approval shortly. Viking Therapeutics (VK2809) and Terns Pharma (TERN-501) are among other companies with clinical trial readouts due in the coming months.
The potential market for an approved NASH therapy is expected to be very large, as there are around 100 million people in the US alone with a fatty liver, many undiagnosed, of whom around 5 million will progress to NASH cirrhosis.