Amplifying the patient voice for better engagement, recruitment and retention

Everyone is familiar with the phrase ‘patient-centricity’ but, where it might once have been seen as a ‘checkbox exercise’, it has gained preeminent status in the era of COVID-19, says Prime Global’s Ross Jackson.
The pandemic has transformed many aspects of our industry and one area that change has come to is patient engagement within clinical trials, where it would now seem unusual were we to not consider the patient voice when it comes to recruiting trial participants.
There are many benefits of including patients at every stage of the drug development process, as well as ways to involve them in the early stages of drug development. It helps to focus the drug development process on its main element – the patient, and it also makes good business sense, too.
In terms of how to include patients, at its very simplest, the act of asking patients what they ultimately want out of a drug is at the core of how to get patients involved. Obviously in many cases this may involve having to temper patients’ desire for a flat-out cure, but it can certainly be valuable to understand which aspects of a particular condition that patients are most concerned about.
Taking things a step further, having the patient voice involved in trial design can help to avoid a situation where there is an onerous burden placed on the patient before they even start on the trial. For example, a recent study I was involved with could have seen participants making three Site Visits of up to 3-4 hours each, even before randomization! Not an ideal scenario for most patients to be faced with.
Inclusivity and trust
There’s a clear logic behind including patients at the early stages of drug development projects. It produces a more consistent and collaborative relationship with patients and results in an easier recruitment process through word of mouth within patient networks, with a greater likelihood of patients remaining on trials for longer.
According to one 2018 study, “feeling valued” was viewed as essential, for patients participating in trials, along with the desire “to feel equally important on the research team, with appropriate recognition and respect”.
Patients also feel a greater sense of inclusivity and, therefore, trust if they are involved with a trial from the beginning. This then has a practical impact on recruitment rates.
"It’s important to see patients as partners and collaborators in the process of drug development"
Various studies have concluded that trials based on listening to the patient voice from the outset are more likely to recruit in a shorter time period than trials that don’t incorporate patient-centricity. In one of these studies, carried out by patient community organisation, Health Union, 66% of chronic disease patients suggested they would be more likely to take part in trials if they knew other patients had been involved from the outset.
Boosting clinical trial recruitment with digital marketing
The key to effective patient recruitment is to reach patients where they are, with the right message. To look at the second part of that first, if you can incorporate a patient-centric element in your message, you will automatically help attract people who might otherwise not be interested.
Where digital marketing is so effective is in reaching patients where they are – essentially, on social media and engaging in other types of online activity, such as browsing websites or video channels. I wrote an Amazon-bestselling book called ‘Patient Recruitment for Clinical Trials using Facebook Ads’ that provides the foundations for how you can reach patients on Facebook. I didn’t choose that platform simply because it’s one I understand how to use, I chose Facebook because it’s consistently proven to be the most effective method for reaching patients ‘where they are’. (And if you’re not on Facebook yourself, you will almost certainly know someone who is – which is where the right messaging can be so effective at encouraging people to share information about trials with people they know).
"Patients feel a greater sense of inclusivity and, therefore, trust if they are involved with a trial from the beginning"
Other digital channels have also proven to be valuable for the purpose of patient recruitment – including YouTube, Snapchat (for a younger audience), ‘small ads’ style websites such as Gumtree or Craigslist, ‘native advertising’ platforms such as Outbrain and Taboola, plus Google can sometimes work OK (though not always), and I’m even aware of some successful trial recruitment having been done through TikTok!
Fundamentally, your potential clinical trial participants are online somewhere, so digital marketing is one of the most effective methods of reaching them. Especially when combined within an overall strategy that involves patients, patient advocacy groups and HCPs throughout the recruitment process.
Reducing time-to-market
Recruiting patients in a timely fashion is one of the industry’s biggest problems, so saving time at this stage helps reduce the overall length of time it takes to get a drug from initial testing to the marketplace. As well as the commensurate cost saving through not having to extend the duration of the trial and change the protocol as a result.
One study found an average saving of 37% in the time it takes to recruit sufficient patients for oncology trials when the patient voice was integrated into the trial design from the outset.
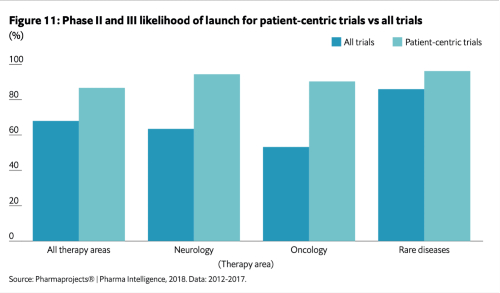
Interestingly, it’s not just the time it takes to get to market that is improved, it’s also the likelihood to getting to market in the first place. In a 2018 study of trials in multiple therapy areas, drugs developed using patient-centric designs were 19% more likely to be launched than drugs developed without incorporating patient-centricity in their design.
Benefits for future clinical trials
Trial participants are giving a lot – in terms of their time commitment, access to personal data, insights and experience, and ultimately with the potential risk to their health. For this reason alone, it’s important to see them as partners and collaborators in the process of drug development, rather than them being the ‘necessary evil’ that maybe characterised some previous approaches to patient care during trials.
Pharma companies such as Pfizer and others recognise the value that patients and patient organisations bring to their research, being experiential subject matter experts in their particular therapy areas. As well as this, government bodies are also becoming more interested in incorporating the patient voice in the regulatory process, with both the FDA and EMA having patient-focused programs and committees.
As momentum builds in this area, we are delighted to be involved with the first-ever annual Patient Engagement Day on 1 September 2021. It’s a chance to celebrate the role of the patient in drug development and amplify the patient voice in clinical trials, which will ultimately result in better outcomes for everyone.
About the author
 Ross Jackson has been working in digital marketing for over 20 years. Early on he developed a specialism in the healthcare sector and has focused on patient recruitment for clinical trials since 2015. As well as digital marketing, his experience with over 100 recruitment projects encompasses dealing with multiple stakeholders in the clinical trials process, from patients and patient groups to individual HCPs, research sites, CROs and sponsors. He is the author of the Amazon bestseller Patient Recruitment for Clinical Trials using Facebook Ads and recently joined Prime Global as their VP of patient recruitment services.
Ross Jackson has been working in digital marketing for over 20 years. Early on he developed a specialism in the healthcare sector and has focused on patient recruitment for clinical trials since 2015. As well as digital marketing, his experience with over 100 recruitment projects encompasses dealing with multiple stakeholders in the clinical trials process, from patients and patient groups to individual HCPs, research sites, CROs and sponsors. He is the author of the Amazon bestseller Patient Recruitment for Clinical Trials using Facebook Ads and recently joined Prime Global as their VP of patient recruitment services.
About Prime Global
Prime Global are experts in medical communications, market access, patient insights, engagement, and recruitment. They help the world’s leading biotech, pharma and healthcare companies to transform global health and patient outcomes, now and for future generations.











