Using bispecific antibodies to advance cancer immunotherapy
Dr Laura Moriarty, senior marketing manager at Bio-Rad, looks at the impressive immuno-therapeutic potential of bispecific antibodies (bsAbs).
Bispecific antibodies (bsAbs) are designed to recognise two different epitopes and, compared to current monovalent antibody therapeutics, bsAbs have enhanced binding, specificity, and efficacy, making them exciting candidates for more targeted cancer treatments. In 2019, there were 57 bsAb candidates in clinical trials, against both haematological and solid tumours.
BsAbs physically link two distinct epitopes in a dependency that can be temporal, with binding events occurring sequentially, or spatial, with binding events occurring simultaneously - a novel functionality which extends the mechanistic range of antibody-mediated therapies, such as linking an effector to a target cell, or engaging two molecules on the membrane of one cell. Though their bispecific nature complicates large-scale production and purification workflows, with challenges such as antibody chain mispairing, bsAbs have come a long way since first developed. They provide clinicians with an off-the-shelf approach to cancer treatment, and they are typically cheaper and easier to produce than cell-based therapies.
Extending the reach of immunotherapy
Compared to haematological cancers, solid tumours have proven more difficult to target with current antibody and cell-based therapies, typically due to lower antigen expression levels and immunosuppressive tumour microenvironments. The more targeted, bispecific functionality of bsAbs broadens the reach of antibody-mediated therapies against both solid and haematological cancers, creating novel approaches that can induce a tumour-specific immune response, target immune checkpoints, or improve payload delivery to tumour cells (Fig. 1).
Clinical interest in bsAbs gained momentum following the promising data and market success of blinatumomab (BLINCYTO®, Amgen), a fragment-based bispecific T cell engager (BiTE), which first received FDA approval in 2014 and EMA approval in 2015. The BiTE platform is an innovative technology designed to engage the immune system against numerous stages and types of cancers. BiTE molecules function by inducing proximity at the cellular level, bringing T cells together with tumour cells to initiate an immune response with a cytotoxic effect. Acapatamab, for example, is an anti-prostate-specific membrane antigen x anti-CD3 BiTE molecule, and is currently being investigated for the treatment of prostate cancer and non-small cell lung cancer.
BiTE molecules offer more versatility in potential cancer treatments than current cell-based therapies. They eliminate the need to extract T cells for manipulation, expanding the accessibility of immuno-oncology to patients and clinical areas currently unmet by cell-based therapies, such as CAR T-cell treatments. They are also designed to have a short in vitro half-life, meaning that they will be cleared from a patient’s system within several hours. In the pipeline, Amgen has BiTE molecules targeting seven types of cancer in clinical trials, as well as further structures in the design stage.
Further to T cell engagement, bsAbs offer promise in targeting immune checkpoints to advance solid tumour therapeutics. For example, one target under clinical assessment is CD47, an immune checkpoint that is upregulated in solid and haematological cancers as an immune evasion technique. CD47 is ubiquitously expressed and, as a result, antibody-mediated therapies to date have been unable to distinguish between CD47-expressing tumour and healthy cells. To overcome this, bsAbs can be designed to have affinity for both CD47 and tumour associated antigens (TAAs), and thus selectively bind CD47-expressing tumour cells.
BsAbs also have the potential to improve the delivery of payloads, an isotype or drug, to tumour cells. Payload delivery using monovalent antibodies, such as radioimmunotherapy and antibody-drug conjugates, relies on the direct coupling of a payload to the antibody, which then binds directly at the tumour site for delivery. However, prolonged exposure of healthy tissue to the drug or isotype can incur adverse, toxic effects. BsAbs that bind both the payload and TAAs eliminate the need for direct coupling and enable pre-targeted delivery, achieved by injecting the bsAb and payload sequentially, to reduce payload exposure to healthy cells. Five bsAbs delivering payloads entered clinical trials in 2019, four of which targeted solid tumours.
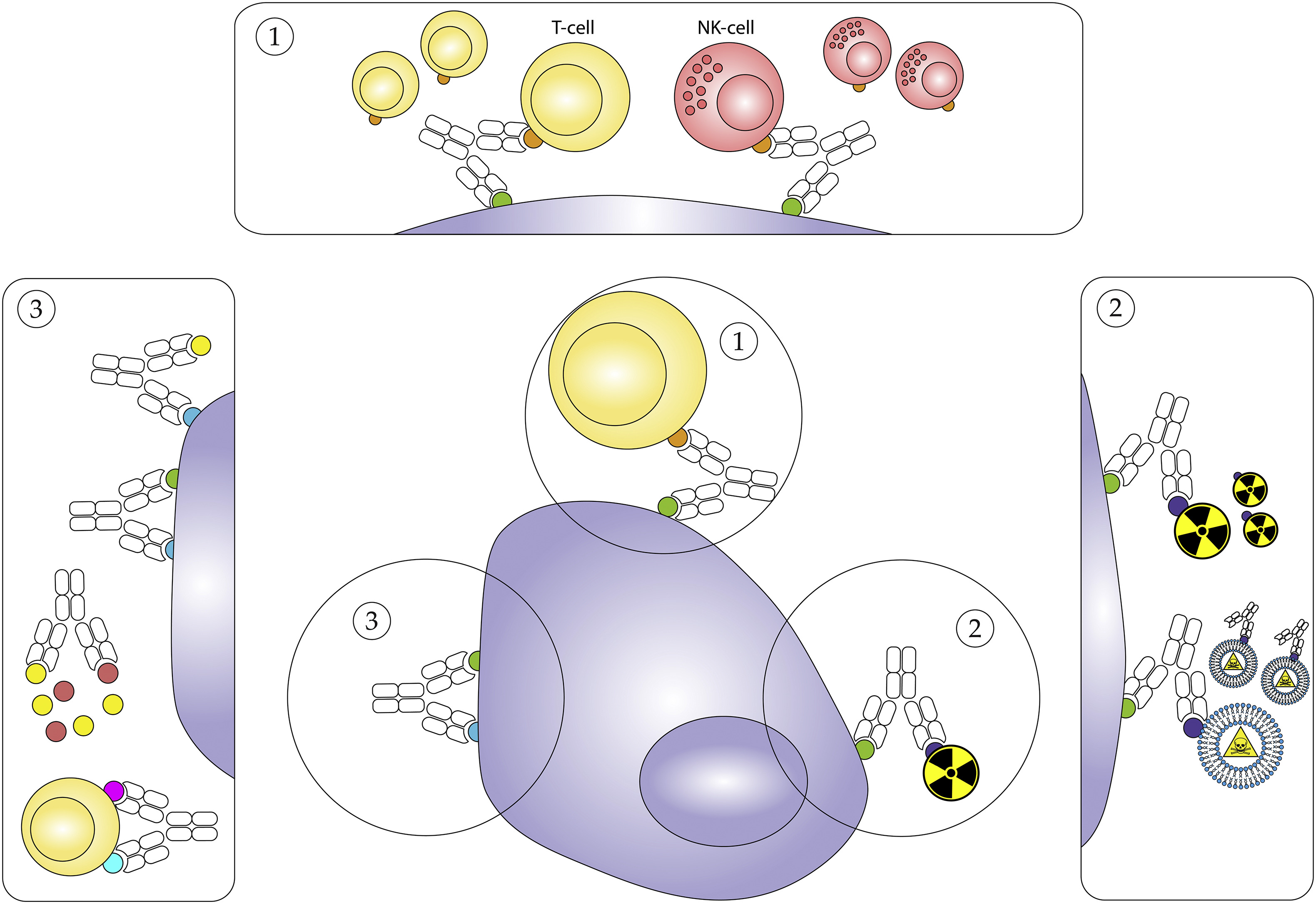
Fig. 1. Simplified schematic overview of the proposed mechanisms of action for bsAbs. 1. Engagement of immune cells to the tumour cell. 2. Targeted delivery of payloads. 3. Targeting immune checkpoint signalling.
Solving the ‘chain-association issue’
The archetypal asymmetric format of bsAbs requires the expression of four unique chains. This format seeks to closely resemble the architecture of native antibodies, to preserve favourable properties such as stability and solubility, as well as lowering the potential for immunogenicity. To obtain a functional asymmetric bsAb, each heavy (H) chain must pair with its cognate light (L) chain, and the two H chains must heterodimerise.
The co-expression of two distinct H and two distinct L chains results in a complex of 16 potential H2L2 combinations, and thus one of the initial challenges in development is obtaining the functional bsAb from this mixture. Though most HL-chain pairs hold a preference for their cognate partner, undesirable mispairings often occur; this challenge is commonly referred to as the ‘chain-association issue’. Using chimeric quadromas, common light chains, and recombinant proteins in bsAb production offers solutions to this issue by limiting the options for association, thus promoting H chain heterodimerisation and forcing cognate HL-chain pairing.
Chimeric quadromas, a hybridisation of two cell types of different species origin, reduce the number of mispaired H2L2 combinations through species-restricted HL-chain pairing. Recombinant proteins - popular for their flexibility regarding origin, composition, and production systems - can be utilised to force the correct association of HL-chains and H chain heterodimerisation, through several mechanisms. For instance, in the ‘knob-in-hole’ approach, one H chain is engineered to consist of relatively large amino acids and the other to consist of relatively small amino acids, to facilitate cognate pairing.
Another approach to solving the chain-association issue lies in the design of alternative bsAb formats. For example, a fragment-based format is a minimalistic approach that combines multiple antigen-binding moieties into one molecule without an Fc region, and thereby circumvents the chain-association issue. This lack of complexity also promotes simpler production workflows, offering the advantage of reduced costs and high yields. An additional bsAb design that alleviates chain mispairing is a symmetric format, which incorporates both specificities in a single HL pair or polypeptide chain. Unlike the fragment-based format, the Fc region is retained, which improves pharmacokinetic properties and effector functions.
Overcoming challenges in large-scale production and purification
Despite the exciting potential for more targeted cancer treatments using bsAbs, the challenges presented in monovalent antibody discovery and development workflows are effectively doubled due to their bispecific nature. Rapid, accurate and highly sensitive screening, production and purification methods are therefore crucial for large-scale bsAb development.
Flow cytometry is a high-throughput screening technique performed directly on antibody-expressing cells, and allows a quick, multiplexed analysis for candidate selection. Once a bsAb candidate is identified, a cell line, such as the previously mentioned chimeric hybridomas, must be used to produce it reliably and consistently. Though advances in genetic engineering have greatly supported high-producing cell lines, the successful development of bsAbs at scale relies on the genetic stability of such lines. Droplet digital PCR (ddPCR) technology provides a sensitive, accurate, and absolute quantification of gene edit confirmation in cell line development, and the gene copy number can be reproducibly determined to assure safety and stability. To improve custom cell engineering workflows, ddPCR offers a screening strategy to characterise the expected homologous recombination frequency, as clones containing heterozygous and homozygous edits can be easily distinguished.
Therapeutic bsAbs also require a highly selective and easy-to-scale system to obtain a suitable purity for the final product. Resin-based chromatography is typically used during antibody purification workflows, and there are several novel modifications to this familiar technique that can improve bsAb purification. For example, mixed mode resins have the unique ability to combine different types of interactions - such as hydrophobic, ion exchange, and metal affinity - into a single support matrix, to improve selectivity, binding capacity, and salt tolerance. Incorporating mixed mode resins into chromatography systems allows bsAbs to be effectively purified according to their distinct epitope affinities, whilst removing product-related impurities to near-negligible levels.
Next steps for bispecific antibodies
There is an increasingly dynamic preclinical and clinical landscape for bsAbs, with a range of novel targets, formats, and mechanisms of action in the pipeline. Their capability to improve treatments for both solid and haematological cancer makes them an exciting addition to the immuno-oncology toolbox. Nevertheless, innovative methods to overcome challenges throughout bsAb discovery and development workflows are essential for their full potential to be realised, and to support continued advances in the field.
About the author
 Dr Laura Moriarty is senior marketing manager at Bio-Rad.
Dr Laura Moriarty is senior marketing manager at Bio-Rad.












