Satsuma pulped as acute migraine drug flunks phase 3 trial
Shares in California biotech Satsuma Pharma have lost three quarters of their value after lead drug STS01 failed to show any improvement over placebo in an acute migraine study.
The drug – a new dry powder intranasal formulation of the old migraine therapy dihydroergotamine mesylate (DHE) – showed a trend towards improvements in pain and other symptoms in the phase 3 EMERGE study, but they didn’t achieve statistical significance.
The failure looks set to scupper Satsuma’s plans to file for approval of STS01 in 2021, and hands a big advantage to rival biotech Impel Neuropharma, which reported positive results with its nasally-delivered liquid DHE treatment INP104 in June, setting up a filing before year-end.
DHE has been used to treat migraine since the 1940s initially as an intravenous therapy and more recently as subcutaneous and intramuscular injections.
The drug has been largely superseded by orally active triptans, but not all patients respond to these and DHE is already available as a liquid nasal spray in Migranal, a drug sold by Bausch Health in the US.
It was also tested as an orally inhaled therapy by Allergan and MAP Pharma, although their formulation Semprana was rejected multiple times by the FDA – for the last time back in 2014.
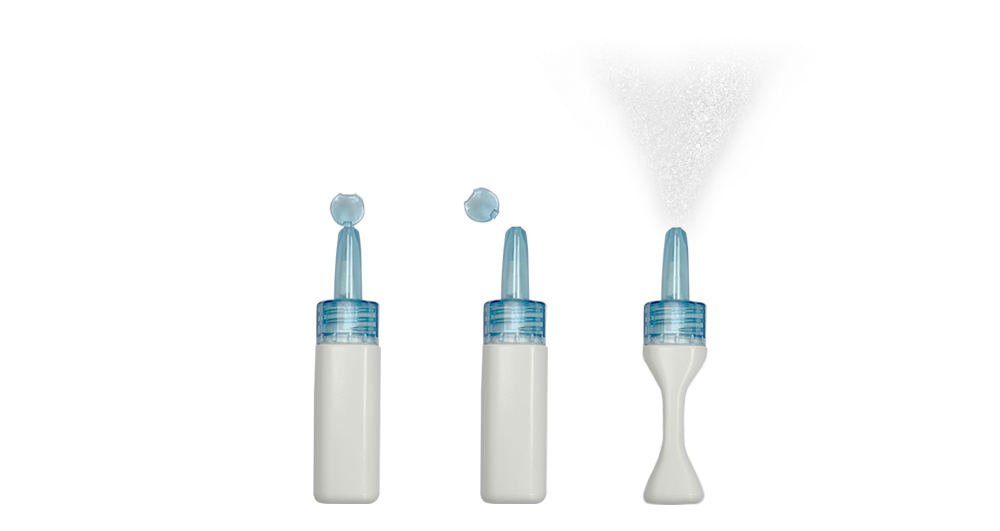
STS01 was designed to overcome limitations with Migranal, including variability between people in drug absorption that means it can take a long time to for the drug to start working.
Satsuma also maintains that Bausch’s drug uses a cumbersome delivery device that requires attachment of a sprayer to a vial and priming of the sprayer before dosing, which can be time-consuming and challenging when a migraine sufferer is in the early stages of an attack.
STS01 takes the form of a prefilled, single-use device that is able to deliver the dose of DHE within seconds, an improvement on Migranal. According to Satsuma it could also be easier to administer than oral triptan drugs as migraine sufferers often have nausea and vomiting as related symptoms.
The EMERGE data undermines all those theoretical advantages, however. In the study, the drug failed to outperform placebo on the two primary measures – freedom from pain and freedom from most bothersome symptom (such as nausea or sensitivity to noise or light) – two hours after dosing.
The failure leaves Satsuma with its lead programme in disarray, and big decisions to make as it looks to the future with around $94 million in cash on hand.
Prior to the EMERGE readout analysts at Evercore had predicted peak sales of $400 million a year for the drug. Now, CEO John Kollins says Satsuma will review the data more closely before evaluating their options.
While the migraine prevention market has seen seismic changes in the last few years with the launch of injectable and intravenous CGRP inhibitors like Amgen’s Aimovig (erenumab), new options for treating migraines once they have started have lagged behind.
A key development came in January this year when the FDA approved the first oral CGRP inhibitor – AbbVie’s Ubrelvy (ubrogepant) – extending the new class into acute treatment of migraine. Data and analytics firm GlobalData estimates annual sales of $486 million for Ubrelvy by 2025.
Also coming through the acute migraine pipeline is AxSome Therapeutics’ orally-active AXS-07 – based on non-steroidal anti-inflammatory drug meloxicam and generic triptan drug rizatriptan – which could also be filed before the end of 2020.












