Rani completes first trial of capsule that could replace injections
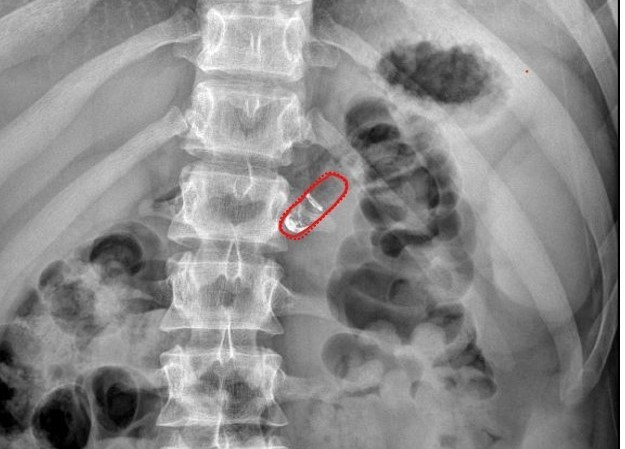
Results of the first human study of a capsule designed to replace injections of biologics have been announced, using an automatic pill designed by Silicon Valley-based Rani Therapeutics.
Backed by Google’s parent company Alphabet, Rani has begun clinical trials of the RaniPill, which is capable of delivering biologic drugs such as insulin and AbbVie’s Humira into the wall of the small intestine.
Rani has already conducted more than 100 animal studies and found the pill showed 100% equivalence with injections.
The RaniPill capsule has a special enteric coating that protects itself in the acidic environment of the stomach.
When the capsule moves into the intestine and pH levels rise, the enteric coating dissolves and a chemical reaction takes place which inflates a balloon.
The pressure in the balloon pushes the dissolvable microneedle filled with the drug into the intestinal wall, where uptake of the drug is immediate due to the high number of blood vessels in the intestinal wall.
Because there are no sharp pain receptors in the intestine, the injection is painless unlike subcutaneous injections.
These can be painful, especially when patients have to inject themselves over and over again.
The RaniPill capsule uses no metal, springs or other elements that the body cannot easily absorb or pass quickly.
Studies in animals and humans have shown they are able to pass the remnants of the capsule within one to four days.
The technology is able to deliver doses of between 3 milligrams, or 3,000 micrograms of the drug, and bioavailability is on par or better than subcutaneous injections.
3,000 micrograms is equivalent to around 80 units of insulin, covering not only pre-diabetic patients but also those with more severe chronic diabetes conditions.
In the trial there were two groups of patients, one of which was fed, while the other fasted.
Each swallowed a drug-free version of the RaniPill capsule in the study testing the capsule’s performance, focused on safety and tolerability.
Results revealed no sensation of the capsule inflating or deploying, and the successful passing of the remnants.
The RaniPill capsule was well-tolerated by both male and female adult subjects of similar BMI with no adverse events.
The study showed similar intestinal deployment times for both fasted and fed subjects, indicating that food does not impact the RaniPill capsule's performance.
A next human study will be conducted later this year and will include a drug-filled resorbable needle.












