Pfizer, BioNTech aim to start German COVID-19 vaccine trial this month
Pfizer and BioNTech are in pole position to start the first human trial of a COVID-19 vaccine in Germany, after getting a green light from the country’s vaccine and biomedicines regulatory body.
The Paul Ehrlich Institut (PEI) gave the go-ahead in just four days for the phase 1/2 trial in 200 healthy volunteers, which will test four coronavirus vaccine candidates, which are based on different RNA formats and target different antigens.
The trials are due to start before the end of this month, said BioNTech chief executive Ugur Sahin at a press conference. First results should become available around the end of June or early July.
Pfizer licensed rights to BioNTech’s BNT162 vaccine development programme last month, sparking a sharp rise in the Mainz, Germany-based biotech’s share price which gradually fell back in the following weeks. The stock was up another 14% today after news of the trial approval was announced.
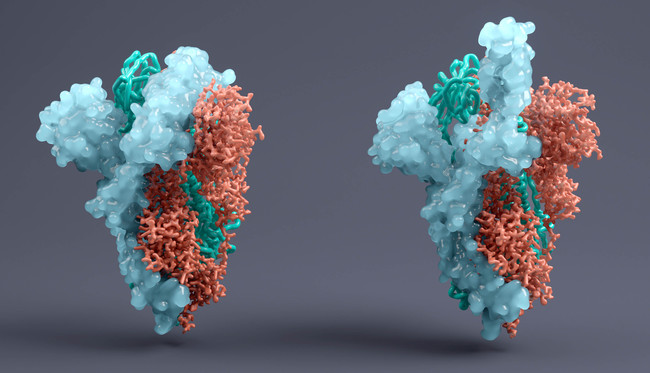
Two of the vaccines are based on nucleoside modified mRNA (modeRNA), one has a uridine containing mRNA (uRNA) structure and the fourth uses self-amplifying mRNA (saRNA), each formulated in lipid nanoparticles.
Two have the full sequence of the spike (s) protein of SARS-CoV-2, the virus which causes COVID-19, and two use a smaller sequence that BioNtech calls an optimised receptor binding domain (RBD) and is thought to be the most important for stimulating antibody response to the virus.
The trial will enrol 200 volunteers aged 18 to 55 and test a range of vaccine doses from 1 µg to 100 µg, gauging safety and tolerability and how well it stimulates an antibody response, whilst also selecting a dose for further studies.
The phase 2 portion of the study will include subjects with a higher risk for a severe COVID-19 infection, according to the PEI.
The first UK trial of a candidate vaccine is due to start today, based on an adenovirus developed at Oxford University, bringing the total number of vaccines in trials to six.
Furthest along in development is Chinese biotech CanSino’s adenovirus-based shot in phase 2, and there are two Chinese candidates based on inactivated SARS-CoV-2 in phase 1, from Sinovac and the Beijing and Wuhan Institute of Biological Products.
US companies have two in early-stage testing – Moderna’s mRNA-based candidate and a DNA plasmid vaccine from Inovio. The World Health Organization’s latest update lists 77 other coronavirus vaccines in preclinical development.
The PEI has also said it expects that “further clinical trials of COVID-19 vaccine candidates will start in Germany in the next few months.”
That could include German biotech CureVac’s mRNA-based vaccine against the coronavirus, which says it is preparing to start a phase 1 trial in the summer, and possibly phase 2 in the autumn.
CureVac was sensationally linked to an alleged attempt by the Trump administration to entice the government to move its programme to the US, which has been strenuously denied by the company.












