Novartis' oral BTK drug moves the needle in CINDU
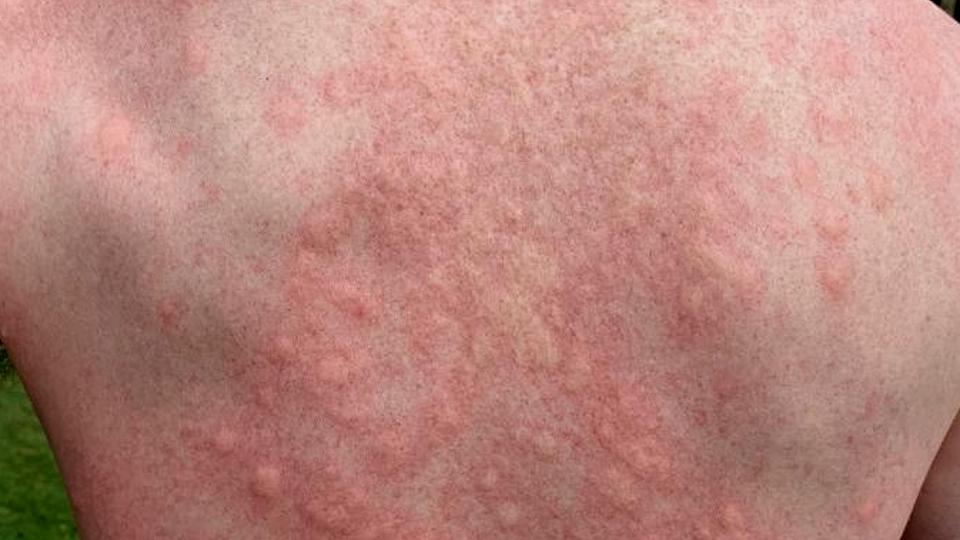
The image shows one form of CINDU, cold urticaria, in a patient who had been swimming in cold water.
Novartis is on course to expand the label for its recently approved oral BTK inhibitor Rhapsido, after chalking up a win in chronic skin disorder chronic inducible urticaria (CINDU).
According to the Swiss pharma group, Rhapsido (remibrutinib) achieves significantly higher complete response rates in the three most common forms of CINDU - symptomatic dermographism, cold urticaria, and cholinergic urticaria – compared to placebo in the phase 3 RemIND trial.
CINDU is a group of conditions, affecting around 0.5% of the global population with 29 million cases worldwide, that is characterised by hives or swelling in response to physical stimuli.
In the case of dermographism, that can be friction or light scratching of the skin, while low temperatures – particularly cold water or wind – are the trigger in cold urticaria. Cholinergic urticaria, meanwhile, can result from exercise, strong emotions, or bathing in hot water.
Rhapsido is already cleared to treat chronic spontaneous urticaria (CSU), in which patients suffer hives and swelling with no known cause and is roughly twice as common as CINDU, with an incidence of around 1% of the global population, according to Novartis.
The company added that it has already filed for approval of the drug for symptomatic dermographism, which is the most common form of CINDU, and will follow up with additional filings around the world – for all three forms – in the coming months.
If approved, Rhapsido could become the first targeted therapy approved for CINDU, which is typically managed by avoiding triggers and the use of second-generation antihistamines, as well as off-label treatment with other drugs, including Roche's anti-IgE antibody Xolair (omalizumab). RemIND enrolled 364 patients whose CINDU symptoms were not controlled using antihistamines.
"Today's findings reinforce that remibrutinib could be the first targeted therapy to improve spontaneous and inducible forms of chronic urticaria, helping address a major gap in care for people living with these conditions," commented Angelika Jahreis, Novartis' head of immunology development.
The new indication could also give Novartis a niche indication where it will not have to compete with Sanofi and Regeneron's huge-selling anti-IL-4 and IL-13 antibody Dupixent (dupilumab), which was cleared for CSU last year, adding to a long list of approved indications.
Dupixent has also been tested in previous clinical trials for CINDU, with initial results reported in cold urticaria. However, Sanofi's latest pipeline listing suggests it is now focusing on another candidate, KIT inhibitor SAR449028, which is in phase 2 testing. It also has an oral BTK drug (rilzabrutinib) in mid-stage testing for CSU.
Rhapsido was approved in the US last September and started rolling out onto the market towards the end of the year. So far, the company hasn't reported any sales figures, but Novartis CEO Vas Narasimhan pointed to "encouraging" take-up in CSU patients refractory to antihistamines, as well as after the failure of biologics, in the company's fourth-quarter 2025 results update.
"Rhapsido is one of these brands that we hope over time could become one of the largest […] in Novartis' history," he continued, saying the early take-up is "right in line with some of the most successful dermatology launches."













