Grail's multi-cancer test fluffs its lines in UK trial

Just weeks after filing for FDA approval of its Galleri blood test for cancer screening, Grail has reported that a flagship trial of the technology in the UK failed to achieve its primary objective.
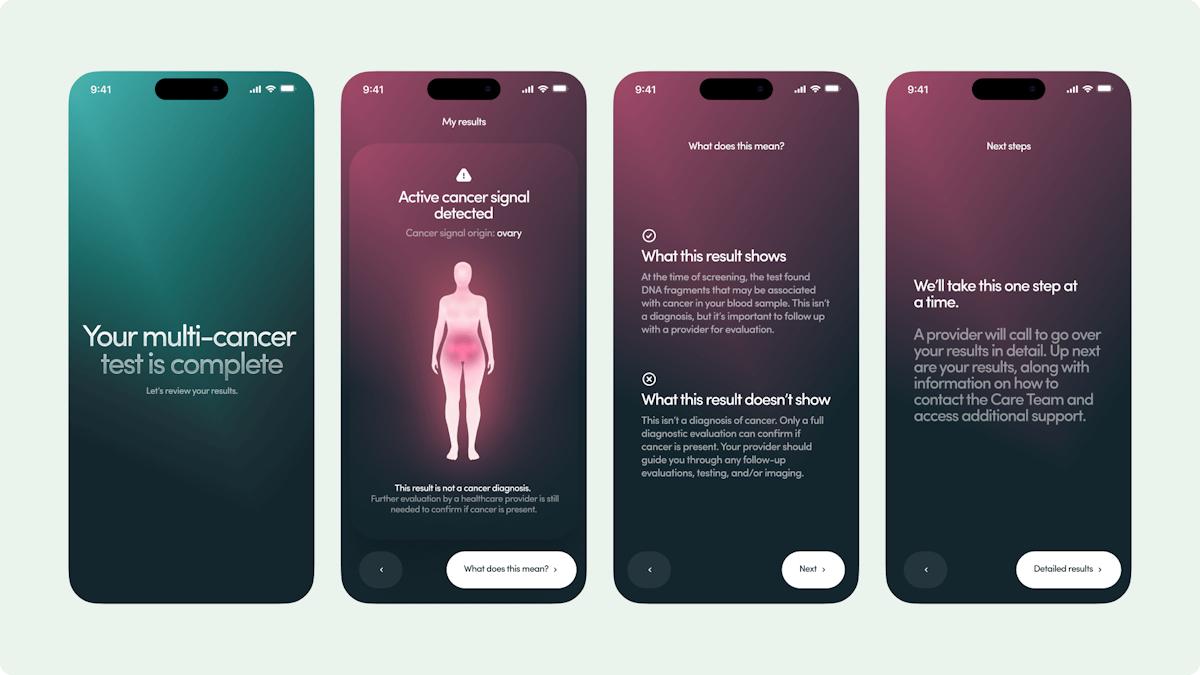
The much-anticipated results of the NHS-Galleri study – which put the multi-cancer early detection (MCED) test through its paces in 142,000 patients over three years – showed that it was unable to reduce stage 3 and 4 cancers among subjects who used it compared to those who did not.
In the study, people aged 50 to 77 with no cancer symptoms provided three blood samples over two years, around 12 months apart.
Shares in Grail were hit hard in after-hours trading, losing around 47% of their value on the Nasdaq, despite efforts by the company to accentuate positive secondary readouts, including a reduction in stage 4 cancer diagnosis, increased stage 1 and 2 detection, and a four-fold increase in the overall detection rate.
The selloff likely stems from Grail's inclusion of metrics from the NHS-Galleri study in its FDA filing, which also draws on interim results of the US-based PATHFINDER 2 study. The company is expecting full data from 35,000 PATHFINDER 2 subjects later this year, and is also enrolling another 50,000-patient study – REACH – in collaboration with Medicare in the US.
The company also said there was a favourable trend in the primary endpoint over time "in a pre-specified group of 12 deadly cancers," which included cancers of the anus, bladder, colorectal, oesophagus, head and neck, liver/bile duct, lung, lymphoma, myeloma/plasma cell neoplasm, ovary, pancreas, and stomach.
The company is now extending the follow-up period of the trial by another six months, in the hope that the trend might firm up and reach the threshold for statistical significance.
Galleri is designed to detect up to 50 different types of cancer in the early stages by detecting chemical changes in fragments of cell-free DNA (cfDNA) that leak from tumours into the bloodstream.
"The NHS-Galleri trial provides the strongest evidence to date that multi-cancer early detection can shift the stage at which cancers are detected at a population level," said Bob Ragusa, Grail's chief executive.
Undeterred by the top-line miss, the company said it is expanding its US sales force and medical teams "to bolster our education efforts and support growing demand," he added.
Direct sales of Galleri, with a prescription, resulted in more than 185,000 commercial sales last year and drove the company's revenues up 26% to $137 million. Earlier this month, it teamed up with telehealth company Hims & Hers to enhance access to the test in the US.