FDA clears sleep apnoea feature for new Apple Watch
Apple has the FDA authorisation it needs to bring its software for detecting sleep apnoea to the Apple Watch, coinciding with the launch of its new operating system for the wearable.
The Breathing Disturbances function is part of watchOS 11, which makes its debut today alongside the release of the latest Apple Watch Series 10 - unveiled at the company's new product reveal earlier this month and due to become available on 20th September.
The FDA cleared the app towards the end of last week via its 510(k) de novo premarket notification pathway, describing it as an over-the-counter device that can provide a notification of the risk of sleep apnoea to people who have previously not been diagnosed with the disorder.
The development comes shortly after Apple also claimed a green light from the FDA to use its AirPods earbuds as OTC hearing aids using its Hearing Aid Feature (HAF) app, the first time that a consumer device has been cleared for that purpose.
Apple confirmed today that the sleep apnoea notification feature will be rolled out to Apple Watch Series 9, Series 10, and Ultra 2 devices.
It uses the accelerometer in the watches to monitor small movements at the wrist during sleep that are associated with interruptions in normal respiratory patterns and, according to Apple, uses an algorithm trained on thousands of nights of clinical-grade sleep apnoea tests to interpret the results.
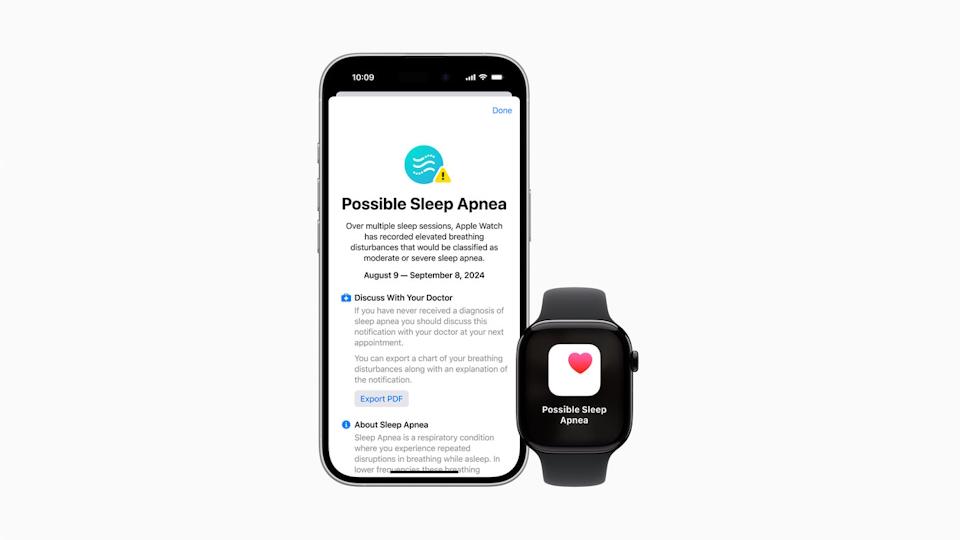
"Users can view their nightly Breathing Disturbances in the Health app, where they are classified as elevated or not elevated, and can be viewed over a one-month, six-month, or one-year period," said the tech giant.
Sleep apnoea is most common in people who are overweight and affects approximately a billion people worldwide. It results in repeated interruptions in breathing during sleep, caused by narrowing and collapse of the airway, and is associated with serious health problems, including hypertension, diabetes, stroke, and heart disease, which can shorten life expectancy.
The FDA's clearance stresses that Breathing Disturbances cannot provide a standalone diagnosis of sleep apnoea or replace traditional methods of diagnosis like polysomnography. It should also not be used to assist in diagnosing sleep disorders or be used as an apnoea monitor.
Apple's position is that the feature can serve as an early warning system for people who may be at risk, encouraging them to speak to their doctor about the next steps, which could lead to diagnosis and treatment.
Users can export a PDF that shows when sleep apnoea may have occurred, three months of breathing disturbance data, and additional information that may be used in discussions with healthcare providers.
It's worth noting that Apple isn't the first company to bundle sleep apnoea functionality into a consumer device. Australian medtech firm ResApp Health also has a smart device app called SleepCheckRx for the disorder, which got 510(k) clearance from the FDA in 2022, shortly before the company was acquired by pharma giant Pfizer for around $115 million.
SleepCheckRx is an at-home test that screens adults for sleep apnoea by analysing breathing and snore sounds during sleep.











