Apple AirPods get FDA okay for use as OTC hearing aids
It's known that excessive use of earbuds at high volumes can cause hearing loss, so somewhat ironic that the FDA has just authorised software that can turn Apple AirPods into hearing aids.
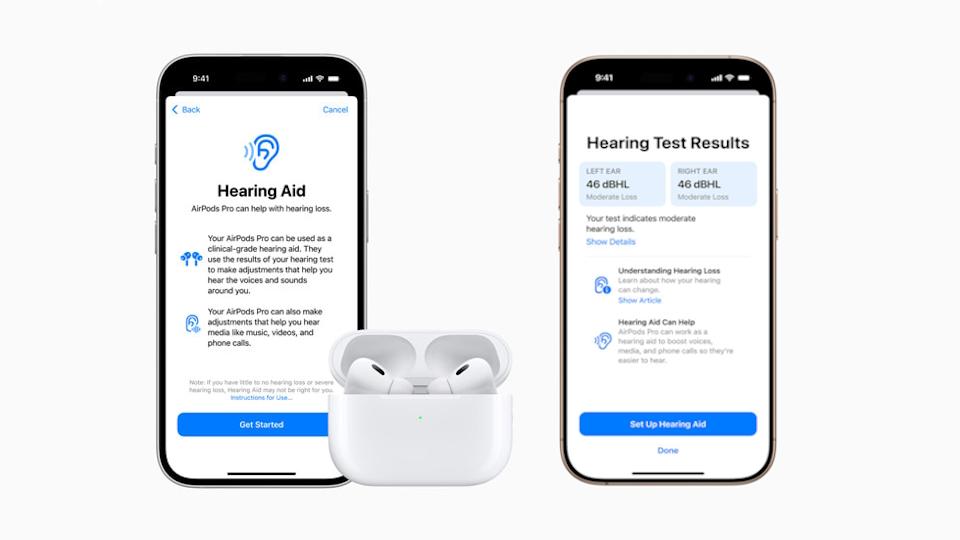
The green light is for Hearing Aid Feature (HAF), an app compatible with Apple's AirPods Pro earbuds that amplifies sounds and can be used for people aged 18 and over with perceived mild to moderate hearing impairment.
At the same time, Apple has made a contribution to protecting hearing with an AirPods feature called Loud Sound Reduction that can dampen down environmental noise levels, working like sophisticated ear plugs that still allow the sounds you want – like music at a concert – to filter through.
In a statement, the FDA said this is the first time it has authorised an over-the-counter (OTC) hearing aid software device and "advances the availability, accessibility and acceptability of hearing support" for adults with hearing problems.
The regulator freed up access to hearing aids two years ago by allowing them to be sold without a prescription for the first time, as part of a drive to address "a significant public health issue impacting millions of Americans," according to Michelle Tarver, director of the FDA's Center for Devices and Radiological Health.
The Hearing Aid Feature is coupled with "clinically validated" Hearing Test software – part of its iOS HealthKit – that sets up the HAF and tailor it to the user's hearing needs in what Apple calls a "self-fitting" approach.
It takes around five minutes to take the test, which delivers results and recommendations to the user as well as an audiogram that can be shared with a healthcare provider.
More than 30 million American adults report some degree of hearing loss, which can have a negative effect on communication, relationships, school or work performance and emotional wellbeing, according to the FDA.
Using hearing aids has been linked to reductions in the frequency or severity of cognitive decline, depression and other health problems in older adults, and the availability of an option on a widely used consumer product – which sidesteps the need for a pricey dedicated device – could be a step forward for public health.
Apple said that a key benefit of the new software is likely to be raising awareness of hearing loss, as it often worsens gradually, leaving many people unaware that they are living with a deficit. It intends to roll out the feature in more than 100 countries and regions, including the US, Germany, and Japan, in the coming months.
The FDA reviewed the HAF under its de novo premarket review pathway, which is used for new low- to moderate-risk devices without a legally marketed precedent.
Apple also recently unveiled a new Breathing Disturbances feature for the Apple Watch designed to warn users if they have sleep apnoea, an interruption in breathing during sleep, which can be a serious condition if left untreated. That feature is also under review by the FDA.












