Amsterdam wins EMA bid after nail-biting tie
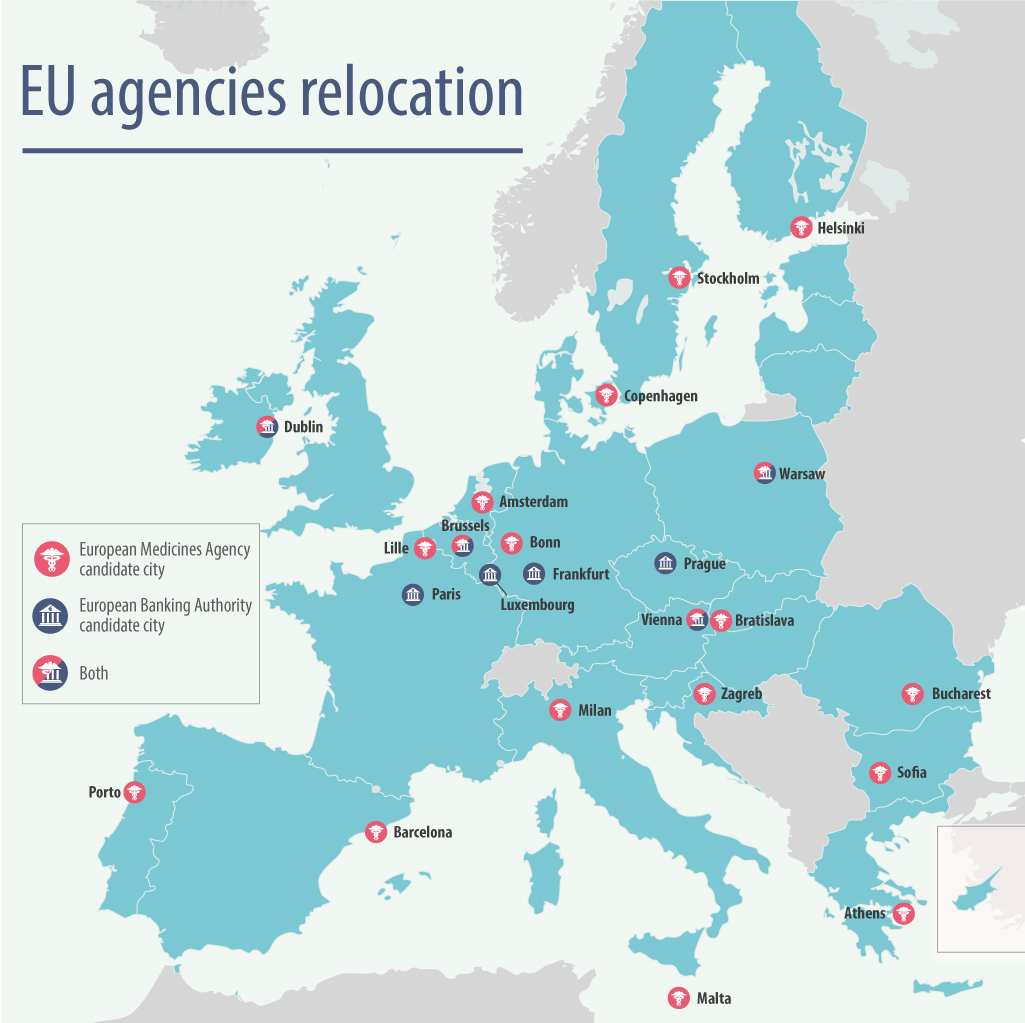
Amsterdam has been named as the new home of the European Medicines Agency after a nail-biting conclusion to voting among the EU27 nations in Brussels this evening.
The Netherlands capital was always one of the frontrunners in the competition to gain the highly prized medicines regulator once it leaves London because of Brexit, but the vote went to a coin toss after Amsterdam tied with Milan on 13 votes each.
This was because Slovakia refused to vote in the latter stages of the ballot, upset that Bratislava had been eliminated from contention, with Copenhagen securing third spot in terms of votes.
Incredible scenes in final round of #EMA voting as Amsterdam and Milan each got 13 votes. Only the drawing of lots could break the tie.
Incredible that a coin toss just determined something that will be pivotal for Dutch power. A big win for the Netherlands. Italians are gutted.
— Dave Keating (@DaveKeating) November 20, 2017
First to respond to the news was the ABPI, the UK pharma industry association which will be very sad to see the regulator leave London.
Commenting on the decision, Mike Thompson, ABPI Chief Executive said:
“Congratulations to Amsterdam on their successful bid. Hosting the EMA is a singular honour for any city and we will do all we can to support the agency’s smooth transition to its new home.
“Up to now the focus has inevitably been on the future location of the EMA. But today’s decision marks the moment when attention should switch to how patient safety and effective public health can be maintained during this complex transition and into the future.
“We now urge both the UK and the EU to put patients first and acknowledge that securing a comprehensive agreement to cooperate on medicines safety, regulation and supply is an urgent negotiating priority.”
Steve Bates, the chief executive of the BIA, the UK's biotech association said: “London’s loss is Amsterdam’s gain. Today’s decision on the location of the European Medicines Agency means a 1000 high quality jobs leaving the UK, disrupting a thousand families as a direct result of Brexit, with implications for thousands more."
He added: "Businesses now need certainty. The best way to do this is by an early agreement to a transition timeframe and continued close regulatory co-operation. We must now ensure Brexit does not disrupt the safe supply of vital medicines to tens of millions of families in the EU 27 and the UK.”
There was of course some disappointment from Italy, which had come so close to securing the agency, and after leading in several rounds of voting.
So after the investments already made, the new site of #EMA, that has an annual budget of over 320M€, moves 60,000 delegates, and decides on more than 500 dossiers, has been decided by the flip of a coin? Europe should have been better than that, but perhaps is not anymore! https://t.co/l88KnB7smi
— Luca Pani (@luca__pani) November 20, 2017
Read more reaction and analysis on pharmaphorum tomorrow, including updates from an EMA press briefing on its plans for relocation and business continuity.











