After trial win, Novartis nabs COVID drug from Molecular Partners
There are still licensing deals to be done in the COVID-19 category it seems, after Novartis paid around $163 million to license rights to an antiviral drug developed by Molecular Partners.
The opt-in has been prompted by the results of a phase 2 trial of ensovibep (MPO420), which showed that a single intravenous dose of the drug was able to not only reduce viral load in non-hospitalised COVID-19 patients over eight days, but also cut the risk of hospitalisation or death by 78% versus placebo.
It's a renaissance for a drug that was all-but written off just a few weeks ago when it failed the ACTIV-3 trial run by the US National Institutes of Health (NIH) in more severely-ill COVID-19 patients who had been hospitalised with the infection.
At the time, Molecular Partners' chief executive Patrick Amstutz said it was possible that the runaway inflammation seen in severely-ill COVID-19 patients meant that they would respond better to anti-inflammatory drugs like Roche's Actemra (tocilizumab) rather than an antiviral therapy.
The new results come from Part A of the EMPATHY trial, which is being conducted in non-hospitalised adults with COVID-19 in the USA, South Africa, India, the Netherlands and Hungary.
Three doses of the drug were tested – 75mg, 225mg and 600mg – and were well tolerated, with the lowest dose being taken forward into the next stage of the trial, according to Novartis.
Novartis is now going all-in on the new drug, announcing this morning that it plans to press ahead with an emergency use authorisation (EUA) application in the US and will also ramp up manufacturing of the drug.
The deal comes amid the first glimmers that COVID-19 may be starting to become less of an emergency – at least in countries with high vaccination coverage that seem to be handling the Omicron wave without their healthcare systems being overwhelmed.
It's early days, but suggests Novartis sees potential for ensovibep in the medium to long term as a highly effective medicine that could be used to treat SARS-CoV-2 after it becomes an endemic infection.
That stems from lab findings which suggest ensovibep maintains its activity against all SARS-CoV-2 variants of concern identified to data, including Omicron.
"These encouraging results come at a time when the need for therapies with pan-variant activity, such as ensovibep, has never been greater," said Amstutz.
If approved it will go up against orally active therapies from Pfizer and Merck & Co, which have also been approved as multi-day courses to treat milder forms of COVID-19 based on data showing they can reduce progression to more severe disease, as well as IV antibodies like GlaxoSmithKline/Vir's sotrovimab and Eli Lilly's bamlanivimab/etesevimab.
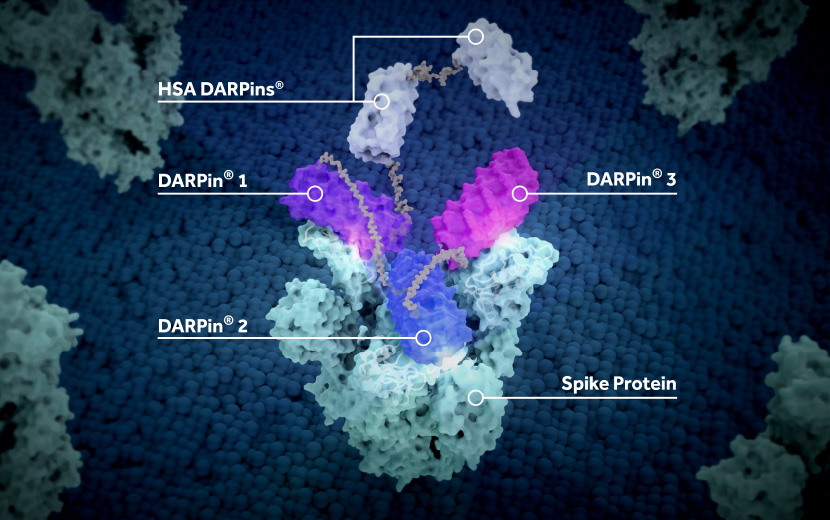
Molecular Partners' drug is designed to block three areas of the receptor binding domains of the viral spike protein, potentially making it less vulnerable to escape mutations that can limit the effectiveness of single-target antibodies.
Ensovibep has the potential to become the first multi-specific antiviral molecule for the treatment of COVID-19, according to Novartis.
The agreement is also another endorsement of Molecular Partners' platform technology, based on genetically engineered proteins known as DARPins, which have antibody-like properties but are much smaller molecules and easier to administer.
Novartis took an option on ensovibep and another DARPin candidate for COVID-19 (MP0423) in October 2020 for an upfront fee of around $65 million, shortly before it reported disappointing results for its Ilaris (canakinumab) antibody in a phase 3 trial.
Amgen and AbbVie are also collaborating with Molecular Partners on DARPin candidates for cancer and ophthalmic diseases, respectively.











