Abbott’s OTC glucose monitor ‘will grow the market’
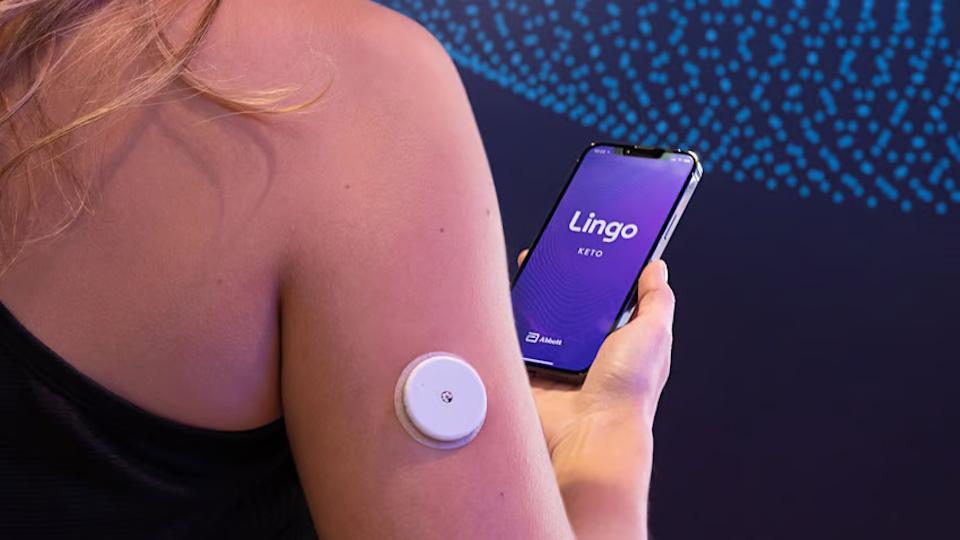
Just a few weeks after Dexcom’s Stelo became the first over-the-counter continuous glucose monitor (CGM) to be approved by the FDA, Abbott has got the green light for its Lingo device - and analysts think uptake by patients is now poised to take off.
Abbott hasn’t issued a public statement on the 510k approval, which has just been published on the FDA’s website, but the wearable device has a similar indication to Stelo and can be used in both non-insulin-dependent type 2 diabetics as well as non-diabetics.
Like Stelo, Lingo takes the form of a patch with a tiny needle that pierces the skin and is worn on the back of the arm for a couple of weeks, communicating glucose readings to a smartphone.
While primarily aimed at diabetics, both Abbott and Dexcom believe there is an OTC market among health-conscious individuals who want to keep tabs on their blood sugar and how it is affected by diet and exercise. Both companies also sell reimbursable prescription CGMs for type 1 and type 2 diabetes, respectively, the G7 and Libre 3 range.
Analysts at William Blair said that with two devices now FDA-approved, demand and market growth look set to “accelerate meaningfully,” given rising numbers of non-insulin diabetics in the US, the broad label for both devices, and the elimination of the need for a prescription.
"Abbott’s entrance should open this market faster than DexCom could on its own," they suggest.
The analysts are predicting compound annual growth rates (CAGR) of CGM devices for insulin-dependent diabetics in the midteen percentage range or better, but reckon the non-insulin market will do even better with 40%-plus CAGR through to 2027.
They also see “significant, longer-term opportunities for CGM, such as for prediabetics and metabolic health use” – the latter category covering for example people who may use the devices to improve nutrition, assist in weight loss, and boost athletic performance.
“We believe there are over 100 million people between prediabetic individuals and potential metabolic health users who add to the [market] that Dexcom and Abbott will be pursuing in the coming years as their next leg of growth,” according to William Blair.
Abbott has already launched Lingo in the UK, where the device seems to be being aimed at the health and wellness market – in line with an approach outlined by the company’s chief executive Robert Ford in 2022 at the CES tech trade show, where he described a range of ‘biowearables’ under the Lingo brand aimed at supporting lifestyle behaviour change.
Based in part on UK pricing for the device, which William Blair says is equivalent to around $1,500 a year, the analysts are modelling that Lingo could become a “multibillion-dollar product for the company over the next decade.” They expect the overall domestic CGM market in the US to reach $7.8 billion in 2025 and more than $10 billion in 2027.