Type 1 diabetes: The importance of early screening and diagnosis
This article was organised, funded, and reviewed by Sanofi.
'Diabetes' as a general term is a condition the public are well-versed in. But, while GLP-1s make the headlines with the possibilities they offer as regards Type 2 diabetes (T2D), it is with a different lens that Type 1 diabetes (T1D) must be viewed, and with a different mindset that symptoms must be treated and managed.
Fundamentally, T1D is an autoimmune disease with no cure, and which cannot be prevented. Although literature suggests shared etiological features between the two types, essentially T1D and T2D (a metabolic disease) are vastly different.1,2
T1D: The treatment landscape, yesterday and today
More than 9 million people live with T1D across the globe.3 It is one of the fastest-growing non-communicable conditions today. The onset of symptoms can be sudden, especially in very young children; indeed, for many years the medical community believed that T1D was only diagnosed in children. However, more than half of the people diagnosed with T1D today are adults.4
Breakthrough T1D UK's Hilary Nathan, Director of Policy & Communications, notes there are two peaks for T1D presenting, between the age of five months and six years, and between nine and 14 years of age. "If you look at the overall prevalence of diabetes, 90% of childhood diabetes is type 1."5
She explains that "in type 1, because it is an attack of the autoimmune system, we can now detect the autoantibodies showing the malfunction of the immune system, and that someone will go on to develop type 1 diabetes."
Pursuit of a cure has been ongoing for over a century now, and "before 1921, a diagnosis of type 1 diabetes would have been fatal," notes Nathan. "People died because their bodies weren't able to produce insulin and, across international partnership of the very best minds, Professors Banting, Best, and MacLeod were able to develop a form of insulin that in 1921 was used to treat their first patient."
"Within two or three months, they were treating wards of children, and type 1 diabetes was no longer the death sentence it once was," continues Nathan. "The discovery of insulin was transformational, and we take that heritage very seriously."
Ahmed Moussa, General Manager of Sanofi UK, agrees: "Working in the field of diabetes for the past 15 years and seeing the scientific advancements that are happening now, I see more and more hope for people living with autoimmune type 1. Currently, HCPs are relying on insulin therapy and any supporting mechanisms, like the continuous glucose monitors, the pumps, the hybrid closed loops [HCLs*], which are very useful. [But,] for the diabetes tech to work, you need to work with the technology, and you need to work on the metabolic manifestation of type 1."
Lessening fear, empowering patients
Throughout 110 years of research into T1D, Sanofi has been one of the main insulin producers globally.
"We believe we can help people living with type 1 to pursue their journey without fear," says Moussa. In November 2023, NICE published guidance that will offer HCLs to around 75% of people living with T1D in England over the next five years.6
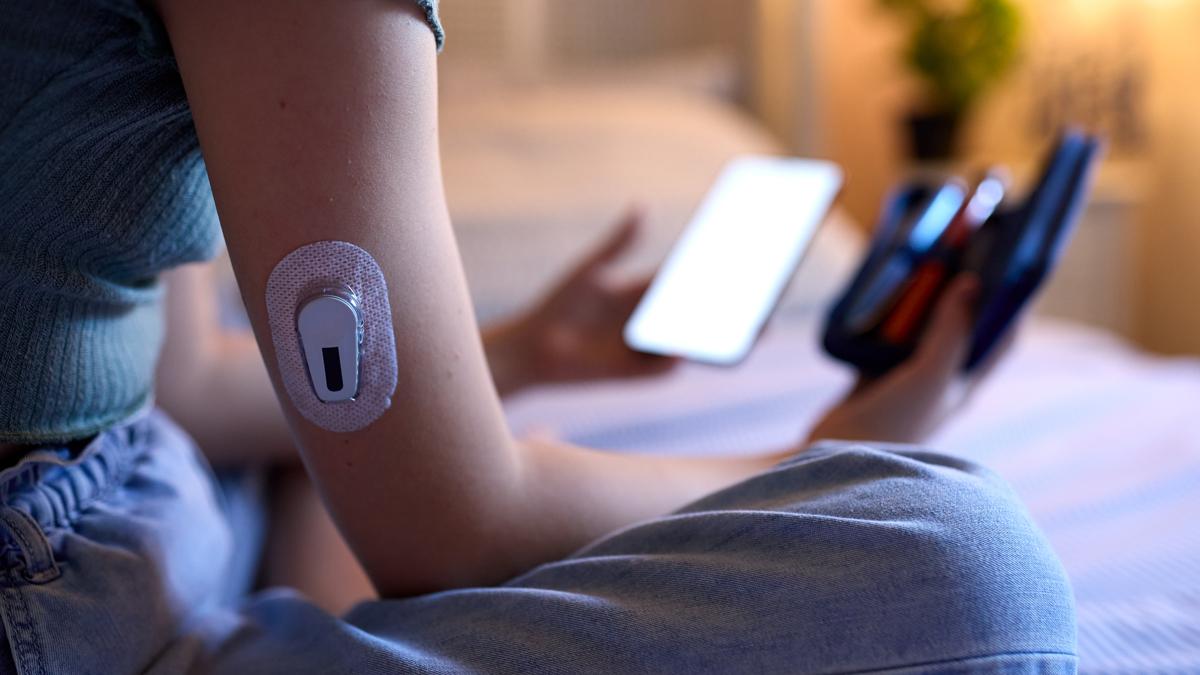
Breakthrough T1D UK funded the research which contributed to the development of continuous glucose monitors (CGMs), introduced commercially over the last 20 years in the UK7 and on the NHS in the last 5 years,8 dependent on nation. These offer an alternative to children and people living with type 1, reducing the need for constant finger pricking.
"Being able to have a pump has meant that people are able to administer the insulin that they need with reduced pain or disruption, rather than the constant injections 10 or 12 times a day," says Nathan. "Hybrid closed loop takes away much of the decision making that people with T1D have to do every day, by automatically administering insulin, meaning people and families with type 1 can sleep through the night, safe in the knowledge that they will get the insulin that they need."
Early detection and screening: The ELSA study
Nonetheless, questions remain about the autoimmune nature of T1D, and to this end, early detection could provide some hope. An effective screening programme for type 1 could transform the way the condition is identified and managed in its earliest stages.
Spotting the signs and symptoms of type 1 diabetes involves remembering what Nathan calls the 4Ts: being thirsty, needing the toilet, being more tired, and becoming thinner.
"If a child is diagnosed with two antibodies, rather than the four antibodies, so diagnosed really early - allowing us an opportunity to get a care pathway in place - we're able to psychologically support families to be in the right place to spot the signs and symptoms," says Nathan.
"Early detection will help to protect people living with autoimmune type 1 from complications and also help families and caregivers in their understanding the nature of the disease," agrees Moussa.
The ELSA study, led by Prof Parth Narendran, Professor of Diabetes Medicine at the University of Birmingham, and co-funded by Breakthrough T1D UK together with Diabetes UK, is screening 20,000 children aged three to 13 years old for T1D.9 A simple finger-prick blood test will reveal their risk of developing type 1 diabetes in the future.
"ELSA is exploring the feasibility and acceptability of recruiting and screening for type 1 diabetes in the general population," explains Prof Narendran. "Screening comes with costs, and a range of psychosocial implications. Understanding the interplay of the cost benefit and psychosocial impact is essential to putting a screening programme forward."
"There are, of course, benefits to screening," he continues. "You can pick up the condition earlier, you can start insulin earlier and reduce the risk of diabetic ketoacidosis. The psychosocial impact in the first year of screen-detected diabetes is lower than if it comes out of the blue, which is how it's traditionally diagnosed."
"They are equipped, and not just living with the shock of a diagnosis, when they first experience type 1 symptoms, enabling timely recognition and intervention," elaborates Moussa. "When you talk to many people living with autoimmune type 1, you understand what is called the 'crash landing' of the disease. Individuals and their families need to adapt quickly to a new lifestyle, to disease-managing daily injection, to regular blood glucose monitoring, and the risk and fear of hypoglycaemia [...] Through early detection and proper education, everyone supporting someone with autoimmune type 1 will be more comfortable as they start this journey together."
Prof Narendran expects less than 2% of the finger-prick tests to be positive for autoantibodies. The team will then give these children a venous blood test, where blood is taken from a vein in their arm. This will happen at a hospital close to the child. This blood test is more accurate than the initial screening test and shows which specific autoantibodies are present in the child's blood.
"In terms of managing the period between being diagnosed with pre-symptomatic type 1 and going on to insulin - that can take many months and sometimes years. There are some guidelines now; international consensus guidelines on how these children and adults should be managed. Publicising that is really important for the UK at the moment," he emphasises. "This is major: just over 100 years after the availability of insulin, we're finally able to move management to a new level of care."
*HCLs are an algorithm that takes blood glucose readings from a CGM and automatically calculates and doses the correct amount of insulin through an insulin pump.
References
- Nyaga, D.M., Vickers, M.H., Jefferies, C. et al. Untangling the genetic link between type 1 and type 2 diabetes using functional genomics. Sci Rep 11, 13871 (2021). https://doi.org/10.1038/s41598-021-93346-x . [Accessed: March 2025]
- Breakthrough T1D UK. What is the difference between type 1 and type 2 diabetes? Breakthrough T1D UK, https://breakthrought1d.org.uk/knowledge-support/about-type-1-diabetes/what-is-the-difference-between-type-1-and-type-2-diabetes/. [Accessed: March 2025]
- Type 1 Diabetes Is Growing Fast and Can Affect Anyone. T1D Index, www.t1dindex.org/. [Accessed: March 2025]
- R. David Leslie, Carmella Evans-Molina, Jacquelyn Freund-Brown, Raffaella Buzzetti, Dana Dabelea, Kathleen M. Gillespie, Robin Goland, Angus G. Jones, Mark Kacher, Lawrence S. Phillips, Olov Rolandsson, Jana L. Wardian, Jessica L. Dunne; Adult-Onset Type 1 Diabetes: Current Understanding and Challenges. Diabetes Care 1 November 2021; 44 (11): 2449-2456. https://doi.org/10.2337/dc21-0770 . [Accessed: March 2025]
- Diabetes. State of Child Health. Royal College of Paediatrics and Child Health, https://stateofchildhealth.rcpch.ac.uk/evidence/long-term-conditions/diabetes/#page-section-3. [Accessed: March 2025]
- Taylor, Phil. "NICE Backs 'artificial Pancreas' Tech for Type 1 Diabetics." PharmaPhorum, 8 Nov. 2023, pharmaphorum.com/news/nice-backs-artifical-pancreas-tech-type-1-diabetics. [Accessed: March 2025]
- Olczuk, D., & Priefer, R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 12(2), 2018, pp. 181-187. ISSN 1871-4021. https://doi.org/10.1016/j.dsx.2017.09.005. [Accessed: March 2025]
- Getting CGM or hybrid closed-loop on the NHS. NHS, https://www.nhs.uk/conditions/cgm-and-hcl-for-diabetes/#:~:text=Getting%20CGM%20or%20hybrid%20closed%20loop%20on%20the%20NHS& text=Ask%20your%20diabetes%20team%20about,people%20who%20need%20them%20most. [Accessed: March 2025]
- The Elsa Study. Elsa Diabetes, www.elsadiabetes.nhs.uk/. [Accessed: March 2025]
About the interviewees
 Prof Parth Narendran is Professor of Diabetes Medicine at the University of Birmingham and The Queen Elizabeth Hospital Birmingham. Prof Narendran's research interest focusses on understanding the autoimmune destruction of insulin secreting beta cells that lead to type 1 diabetes and exploring how this process can be modulated. He is part of a national effort to explore the feasibility and acceptability of early surveillance programmes for pre-type 1 diabetes and is also exploring which therapies are best tested in the prevention arena. He has developed an international reputation for exploring whether exercise can be used to modulate the autoimmune process in type 1 diabetes, and he has recently taken up the Chair of the MS Prevention Taskforce for the Multiple Sclerosis Society to explore treatment parallels across other conditions with similarities to type 1 diabetes.
Prof Parth Narendran is Professor of Diabetes Medicine at the University of Birmingham and The Queen Elizabeth Hospital Birmingham. Prof Narendran's research interest focusses on understanding the autoimmune destruction of insulin secreting beta cells that lead to type 1 diabetes and exploring how this process can be modulated. He is part of a national effort to explore the feasibility and acceptability of early surveillance programmes for pre-type 1 diabetes and is also exploring which therapies are best tested in the prevention arena. He has developed an international reputation for exploring whether exercise can be used to modulate the autoimmune process in type 1 diabetes, and he has recently taken up the Chair of the MS Prevention Taskforce for the Multiple Sclerosis Society to explore treatment parallels across other conditions with similarities to type 1 diabetes.
Prof Narendran leads the Diabetes Research Unit and the Type 1 Diabetes clinical service at the Queen Elizabeth Hospital. He is the author of over 100 peer-reviewed publications, including publications that have been cited by international bodies on Type 1 diabetes and its management. He has served on the Diabetes UK Research Committee and was previously on the Research Advisory Board of the Diabetes Research and Wellness Foundation and Regional Advisory Committee for the NIHR RfPB programme. He chaired the Academic subcommittee of The Association of British Clinical Diabetologists, stepping down in 2023 having led and supported the setting up of its first research grant funding scheme and national Diabetes Update training programme for doctors specialising in diabetes and endocrinology. Prof Narendran reviews for all the major national and international diabetes journals. He contributes to the NIHR Horizon Scanning, and NICE Medical Technology reviews for new therapies.
 Hilary Nathan is Director of Policy, Communications & Community Engagement at Breakthrough T1D. She is responsible for driving regulatory access to T1D treatments in the UK and parliamentary influence. In 2024, Breakthrough T1D UK won the UK's Health & Medical Research Charity Award for its work to secure hybrid closed loop access on the NHS, the most significant advancement in treating type 1 diabetes since the discovery of insulin 100 years ago. She led, co-authored, and launched the 2024 UK Parliamentary Inquiry into Type 1 Diabetes and Disordered Eating, chaired by the Rt Hon Theresa May and Sir George Howarth. With a 25-year career in non-profit management and leadership, Nathan's previous roles include Policy and Research Director at the National Union of Students and Communications Director at The Fable Bureau Marketing Consultancy.
Hilary Nathan is Director of Policy, Communications & Community Engagement at Breakthrough T1D. She is responsible for driving regulatory access to T1D treatments in the UK and parliamentary influence. In 2024, Breakthrough T1D UK won the UK's Health & Medical Research Charity Award for its work to secure hybrid closed loop access on the NHS, the most significant advancement in treating type 1 diabetes since the discovery of insulin 100 years ago. She led, co-authored, and launched the 2024 UK Parliamentary Inquiry into Type 1 Diabetes and Disordered Eating, chaired by the Rt Hon Theresa May and Sir George Howarth. With a 25-year career in non-profit management and leadership, Nathan's previous roles include Policy and Research Director at the National Union of Students and Communications Director at The Fable Bureau Marketing Consultancy.
 Ahmed Moussa is General Manager of General Medicines UK and Ireland at Sanofi, a leading global healthcare company. With over 15 years of experience in the pharmaceutical industry, Moussa has demonstrated a profound commitment to advancing patient care through innovative solutions and strategic leadership. In his current role, he is responsible for overseeing the operations and strategic direction of Sanofi's general medicines portfolio across both markets. His expertise encompasses a wide range of therapeutic areas, with a particular focus on enhancing access to essential medications and improving health outcomes for patients. Prior to joining Sanofi, Moussa held various senior positions within prominent pharmaceutical organisations, where he successfully led cross-functional teams and implemented initiatives that drove growth and operational excellence. He holds an MBA from a prestigious institution and is known for his analytical approach to problem-solving, as well as his ability to foster collaborative relationships within diverse teams.
Ahmed Moussa is General Manager of General Medicines UK and Ireland at Sanofi, a leading global healthcare company. With over 15 years of experience in the pharmaceutical industry, Moussa has demonstrated a profound commitment to advancing patient care through innovative solutions and strategic leadership. In his current role, he is responsible for overseeing the operations and strategic direction of Sanofi's general medicines portfolio across both markets. His expertise encompasses a wide range of therapeutic areas, with a particular focus on enhancing access to essential medications and improving health outcomes for patients. Prior to joining Sanofi, Moussa held various senior positions within prominent pharmaceutical organisations, where he successfully led cross-functional teams and implemented initiatives that drove growth and operational excellence. He holds an MBA from a prestigious institution and is known for his analytical approach to problem-solving, as well as his ability to foster collaborative relationships within diverse teams.
About Sanofi
We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people's lives. Our team, across some 100 countries, is dedicated to transforming the practice of medicine by working to turn the impossible into the possible. We provide potentially life-changing treatment options and potentially life-saving vaccine protection to millions of people globally, while putting sustainability and social responsibility at the center of our ambitions.
Sanofi is listed on EURONEXT: SAN and NASDAQ: SNY
Date of preparation: March 2025
Job Code: MAT-XU-2403764 (v 1.0)












