NICE and orphan drug recommendations: Should we be more worried about non-submissions?

Leela Barham uses the very latest data – running up to November 2023 – to take stock of NICE’s Single Technology Appraisal (STA) recommendations on orphan drugs versus non-orphans. Recommendations since 2018/19 differ only a little between orphans and non-orphan drugs, but non-submissions are typically far higher, limiting patient access.
Research on NICE and orphan drugs
Rare diseases are a focus for many – for example, the ABPI’s chief executive, Richard Torbett, focused on rare diseases in a #MyYearInMedicine highlight - and plenty of research has explored the approach that the HTA body, NICE, takes to rare disease treatments that have secured an orphan drug designation. That includes when orphan drugs go through the Single Technology Appraisal (STA) route; the route that most take.
NICE has adapted its approach for orphans, but access is still delayed
Analysis of NICE’s STAs for orphan drugs has found that NICE has been more flexible and pragmatic in their approach when assessing orphan drugs, yet, it does take the agency longer to reach their recommendations than for non-orphans. Despite changes made to the way that NICE assesses medicines, published in 2022, there have been doubts raised that these are enough to counter delays in access to orphan drugs.
Similar recommendations for orphans and non-orphans
Interest in rare disease treatments is also seen amongst politicians in England. Chris Green, Member of Parliament for Bolton West, tabled a question in Parliament on the 16th October 2023 asking about the recommendations made by NICE under STA for rare disease treatments from 2018/19 to date.
Will Quince, Minister of State for Health and Secondary Care until 13th November, provided data to answer that question. A freedom of information request response from NICE provided more details and updated the data, covering 2018/2019 to 30th November 2023. (It also corrected the data provided to parliament.)
The NICE data illustrates that from 2018/19 to 30th November, the recommendations that NICE made proportionately for non-orphans and orphans were quite similar (Figure 1).
Figure 1: Cumulative NICE STA recommendations, non-orphan and orphan drugs, 2018/19 to 30th November 2023
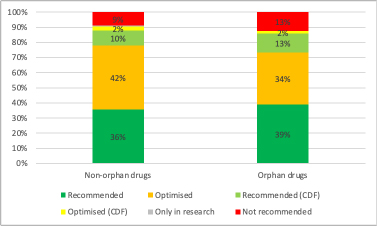
Source: Analysis of data supplied by NICE in response to a freedom of information request. Excludes non-submissions. n = 282 for non-orphan drug recommendations, n = 64 for orphan drug recommendations.
Increasing differences in recommendations?
Cumulative data hides a stark difference for 2023/24, though; comparing recommendations between orphans and non-orphans for the 2023/24 financial year to 30th November shows that NICE did not recommend 40% of orphans, compared to 19% of non-orphans (Figure 2). Whether that’s NICE being tougher or orphans coming to NICE having less compelling evidence and/or poorer cost-effectiveness needs much more detailed analysis.
Figure 2: NICE STA recommendations by financial year, non-orphan and orphan drugs, 2018/19 to 30th November 2023
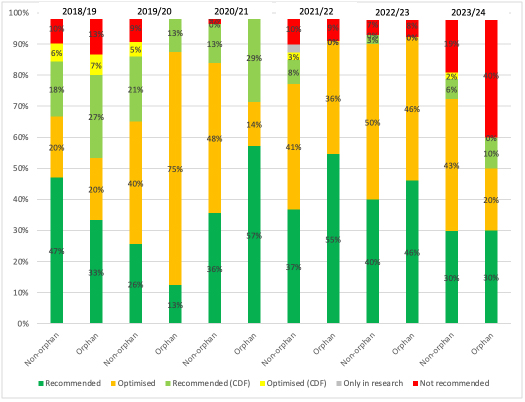
Source: Analysis of data supplied by NICE in response to a freedom of information request. Excludes non-submissions.
Non-submissions differ most
Perhaps more worrying is that there is often – not always, though – a higher proportion of annual NICE recommendations that are classified as terminated for orphans than for non-orphans (Figure 3). That happens when companies decide not to submit.
Figure 3: Number and proportion of non-submissions to NICE, non-orphan and orphan drugs, 2018/19 to 30th November 2023
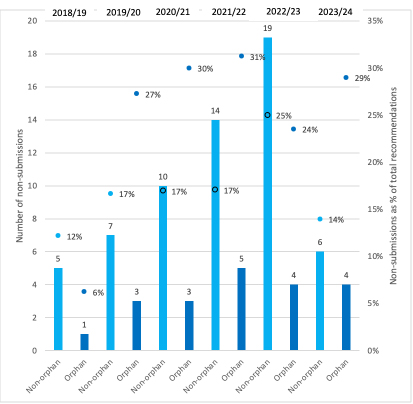
Source: Analysis of data supplied by NICE in response to a freedom of information request. Bars denote the number of non-submissions, dots the proportion of the total NICE recommendations made.
The precise reasons not to submit will only be known by the companies, but for patients and clinicians, their options for access become very limited. They are left with seeking individual funding on an exceptional basis or seeking compassionate access from companies. They may get access if there are still trials ongoing in the UK, too. But routine funding is not an option when companies decide not to put forward their treatment to NICE.
Monitoring required
Time will tell if non-submissions continue, for both non-orphans and orphans. Given that the orphan designation itself implies an unmet patient need, non-submissions are a concern. Stakeholders should monitor and, if the trend in non-submissions persists, explore the underlying drivers, in the hopes of finding a solution.













