Regeneron to develop long release Eylea successor
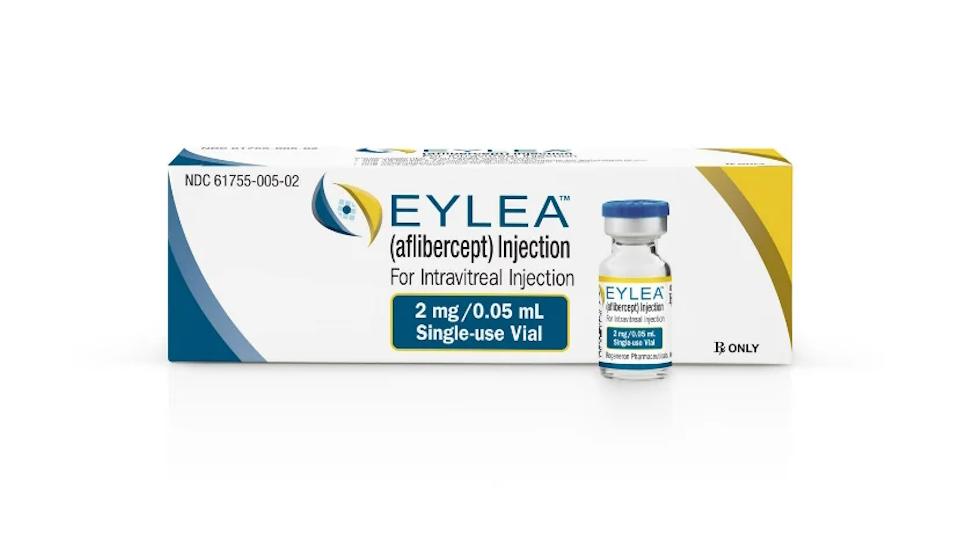
Regeneron has teamed with US biotech Ocular Therapeutix to develop a sustained release successor for its big-selling Eylea, for wet age-related macular degeneration and other serious retinal diseases.
The new formulation of aflibercept is currently in preclinical development – but the drug is approved in the US and Europe as a four-weekly injection under the brand name Eylea.
Marketed in partnership with Bayer, which has ex-US rights, Eylea has become a blockbuster for the companies – but the generous terms of the agreement with Ocular suggest that Regeneron thinks there is more to come from the extended release formulation.
Eylea competes in wet age-related macular degeneration (AMD) with Novartis’ Lucentis (ranibuzumab), and in some cases Roche’s Avastin (bevacizumab), in an unapproved use.
Based in Bedford, Massachusetts, Ocular Therapeutix is developing sustained-release, hydrogel-based drug delivery depots that can be injected into the eye, which can be used with both small and large molecules.
They could be used with small tyrosine kinase inhibitors and larger protein-based and anti-vasular endothelial growth factor traps such as aflibercept, the company said.
Ocular and Regeneron aim to develop a sustained release version of aflibercept that is suitable for clinical development.
Regeneron has the option to obtain an exclusive licence to use the technology for development and marketing of sustained release aflibercept and other biologics, targeting vascular endothelial growth factor (VEGF) for ophthalmic indications.
Upon exercising the option, Ocular Therapeutics would receive $10 million from Regeneron and would be responsible for funding development through phase 1.
Regeneron would be responsible for any subsequent development and marketing costs. Ocular Therapeutix would be eligible to receive up to $305 million in milestone payments for the sustained release version of aflibercept.
This includes $155 million in development and regulatory milestones, $100 million for the first commercial sale and up to $50 million in commercial milestone payments.
Ocular is eligible to receive tiered high single-digit to low-to-mid teen-digit royalties on potential future net sales.