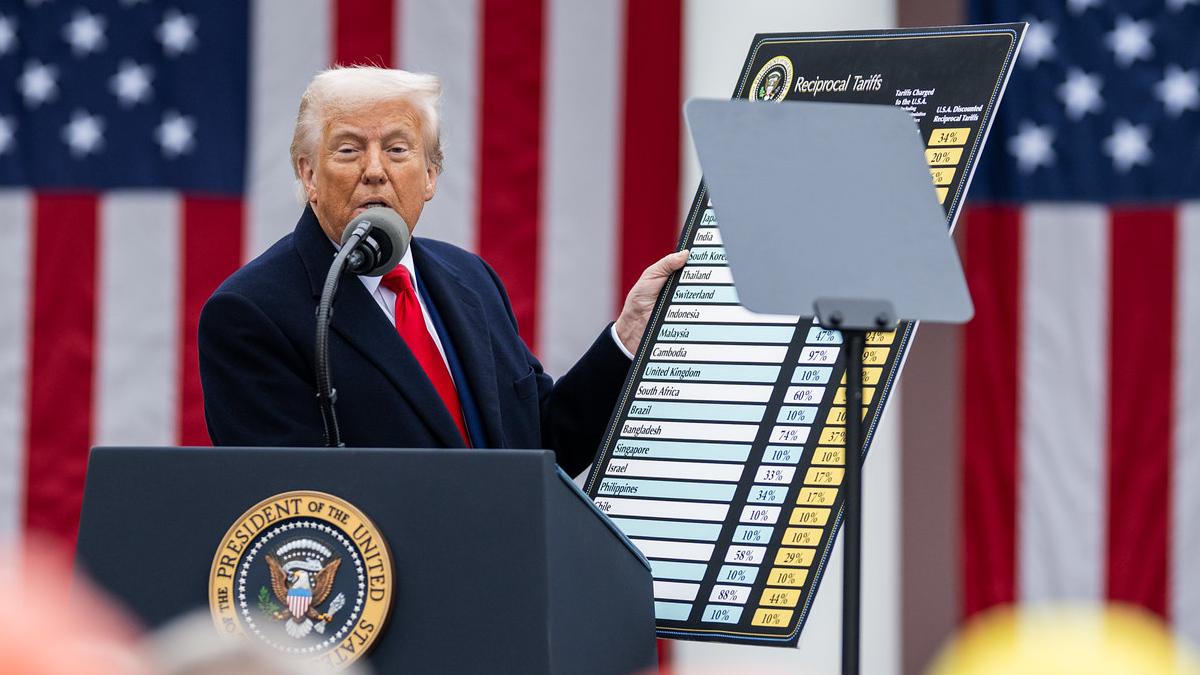
US accuses Regeneron of fraud in Eylea price reporting
The US government has filed a lawsuit against Regeneron, claiming that the company falsely inflated the average sales price for ophthalmology blockbuster Eylea to manipulate the Medicare reimbursement process.
The complaint contends that Regeneron submitted falsified average sales price reports to the Centers for Medicare and Medicaid Services (CMS) that excluded certain price concessions, such as reimbursing drug distributors for credit card processing fees.
It claims that Regeneron paid “hundreds of millions of dollars to subsidise Eylea purchases” that should have been included in its CMS reporting, inflating the reimbursements it received from Medicare and giving it an unfair competitive advantage in the market.
Court documents suggest that Regeneron paid $250 million in credit card fee reimbursements for Eylea purchases to just one of its distributors between 2012 and 2021.
“Regeneron paid these credit card fees so that distributors would accept credit cards for Eylea purchases while still charging a lower, cash price for the drug, and so that Regeneron’s customers – typically retina and ophthalmic practices – could receive credit card benefits for their purchases, such as ‘cash back’ and other credit card rewards,” according to the Department of Justice.
Eylea (aflibercept) has been on the US market since 2011 as a treatment for a range of ophthalmic diseases, including neovascular or ‘wet’ age-related macular degeneration (AMD), with sales of $5.9 billion last year in the US, out of total company revenues of $13.1 billion.
Medicare Part D – a voluntary outpatient prescription drug benefit – paid more than $25 billion for Eylea between 2012 and 2023, according to the government.
“Regeneron greatly inflated the costs of its drug to Medicare over many years and enhanced its revenues,” said acting US attorney Joshua Levy for the District of Massachusetts. “Falsely reported average sales prices cost the Medicare system hundreds of millions of dollars and we will make every effort to prevent such practices.”
Regeneron, meanwhile, said the allegations were without merit and that its reimbursement of costs incurred by specialty distributors was lawful, adding that it plans to “vigorously defend itself in court.”
The case has been brought under the whistleblower provisions of the False Claims Act (FCA) and could allow the government to recoup three times the amount of its losses plus penalties.
It is not the first time that Regeneron has been taken to task for alleged infractions under the FCA concerning Eylea. An ongoing lawsuit, first filed in 2020, claims that the company acted illegally by using a charitable foundation as a conduit to fund co-payments of thousands of Medicare patients.