Merck & Co. drops osteoporosis drug
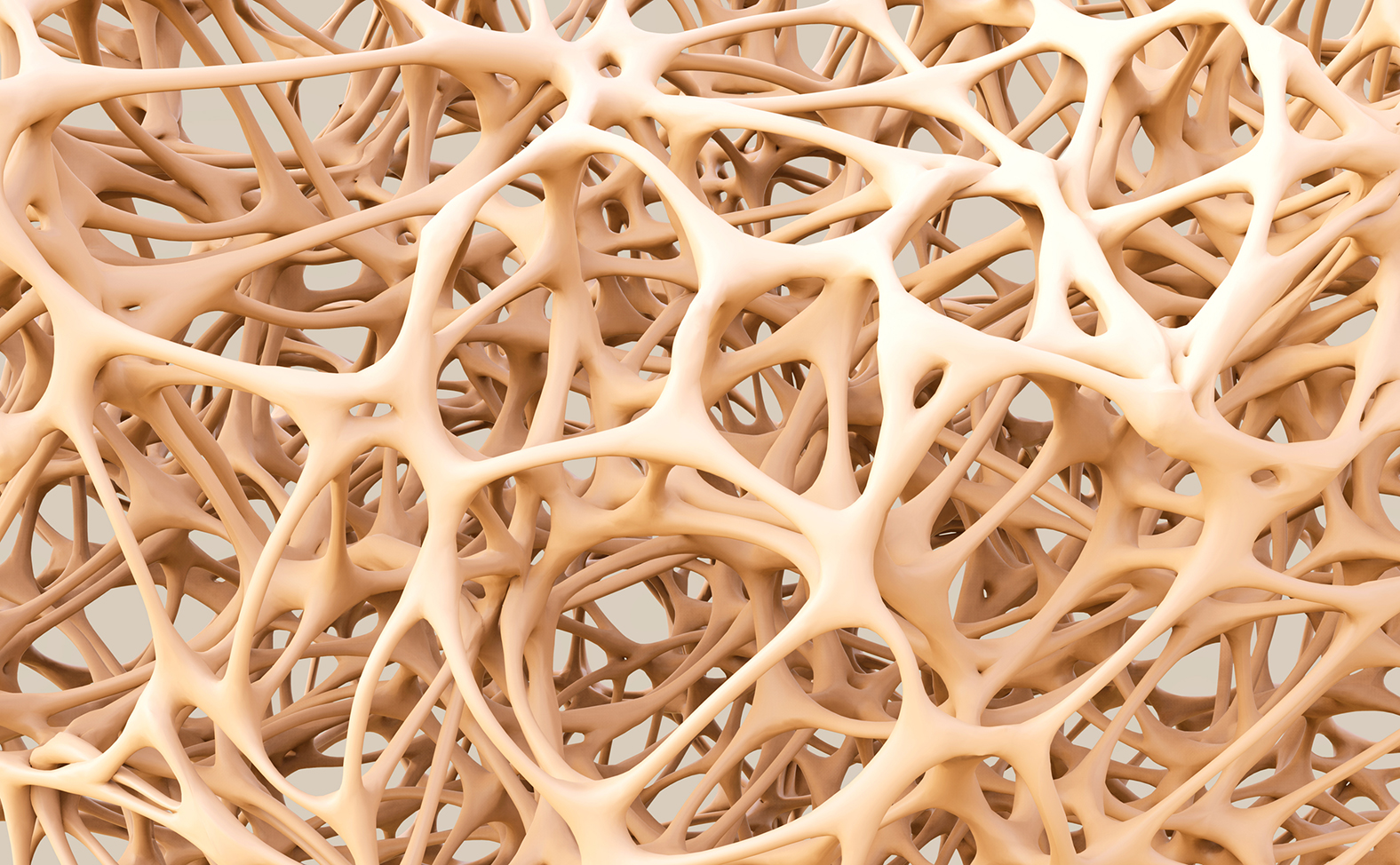
Merck & Co has dropped its odanacatib osteoporosis drug and will not seek a filing, after phase 3 results showed an increased risk of stroke.
The results have boosted shares in Amgen and UCB, which are working together on a rival treatment.
The US company had previously reported a 'numerical imbalance' in adjudicated stroke events in the pivotal phase 3 fracture outcomes study in postmenopausal women.
Now the company has decided to discontinue development after an independent adjudication and analysis of major adverse cardiovascular events confirmed an increased risk of stroke.
Once Merck’s most valuable pipeline asset, shares was once fancied as a potential blockbuster.
But Merck already delayed a filing in 2013 for the drug, which was ironically being developed as a safer alternative to the bisphosphonates commonly used to treat osteoporosis, which can cause osteonecrosis of the jaw.
The market was clearly unsurprised by the news, given Merck’s previous announcements about safety of odanacatib, a cathepsin K inhibitor.
Shares in Amgen and UCB ticked up following the news, as they are developing a rival osteoporosis treatment, romosozumab, in phase 3.
This has a different mechanism and works by blocking the enzyme sclerostin, which like cathepsin K down-regulates formation of bone.
Roger Perlmutter, president of Merck Research Laboratories, said: “We are very thankful to the researchers and patients who participated in the odanacatib clinical development programme.
“We have learned that odanacatib treatment reduces the risk of osteoporotic fractures. At the same time, we believe that the increased risk of stroke in our Phase 3 trial does not support further development.”












