News
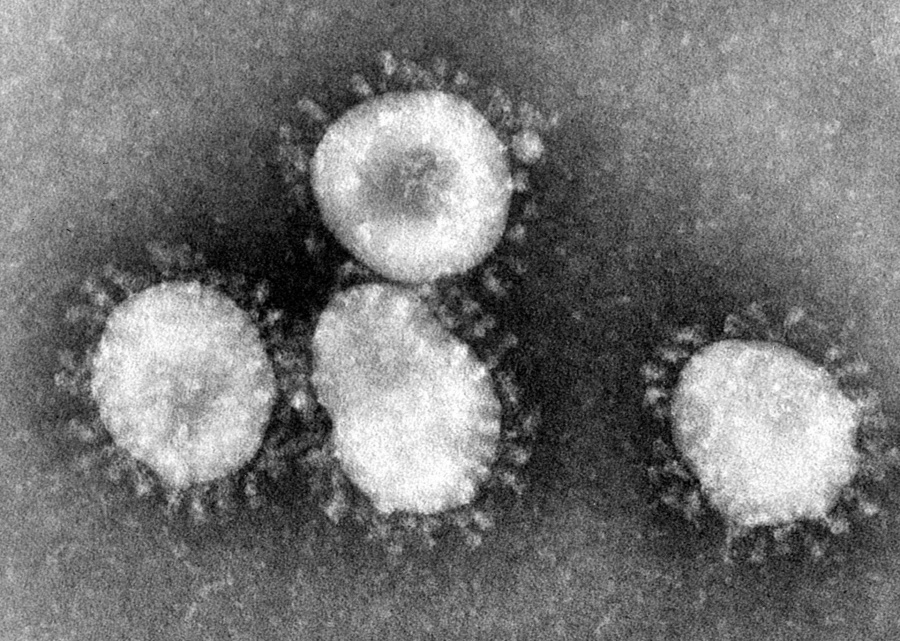
Romark eyes FDA okay for COVID antiviral despite mixed trial...
A phase 3 trial of Romark's antiviral drug NT-300 has missed the main objective in a phase 3 trial in mild to moderate COVID-19 patients, but could still have a shot at emergency use author



